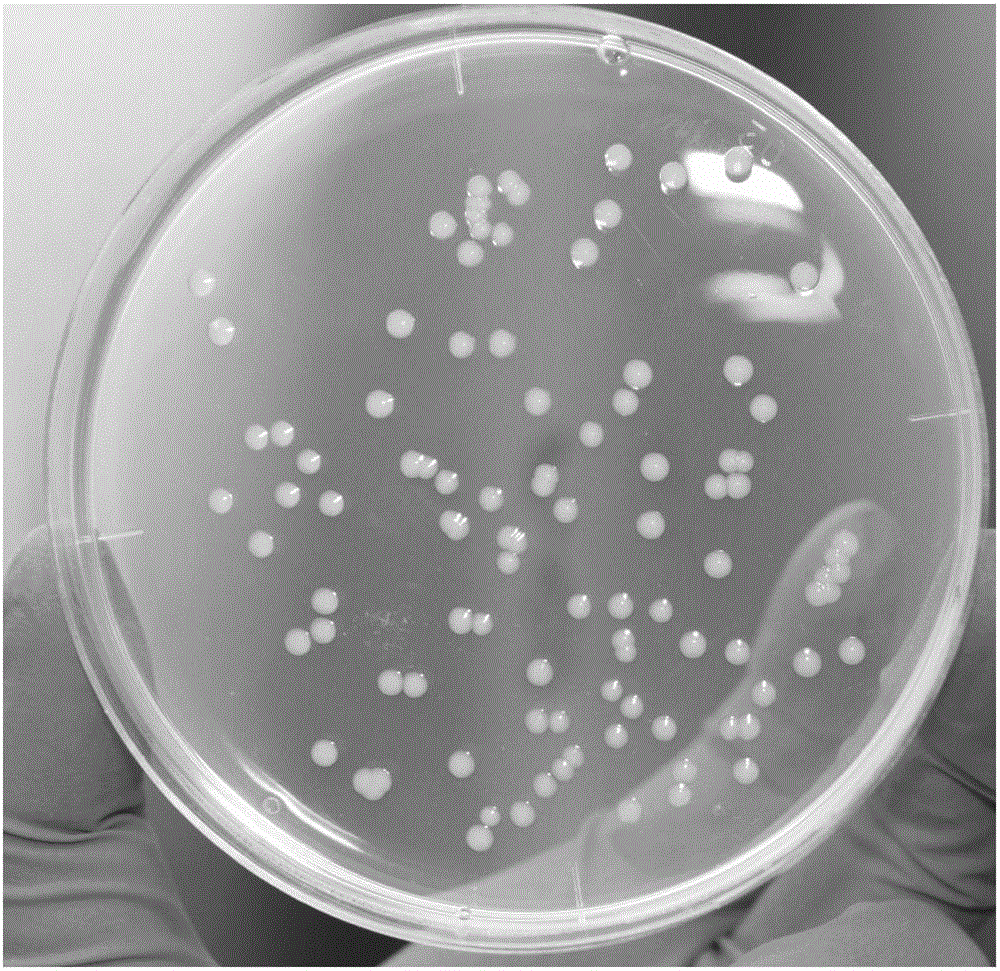
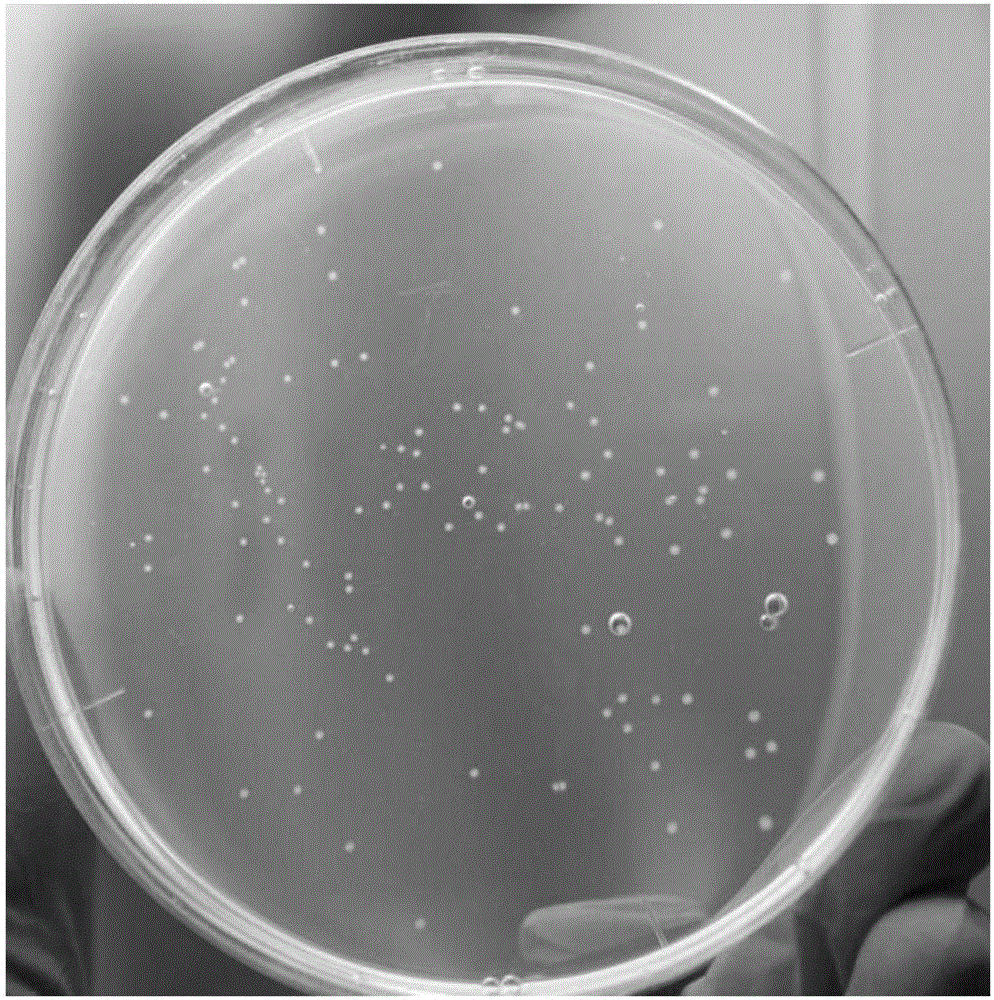
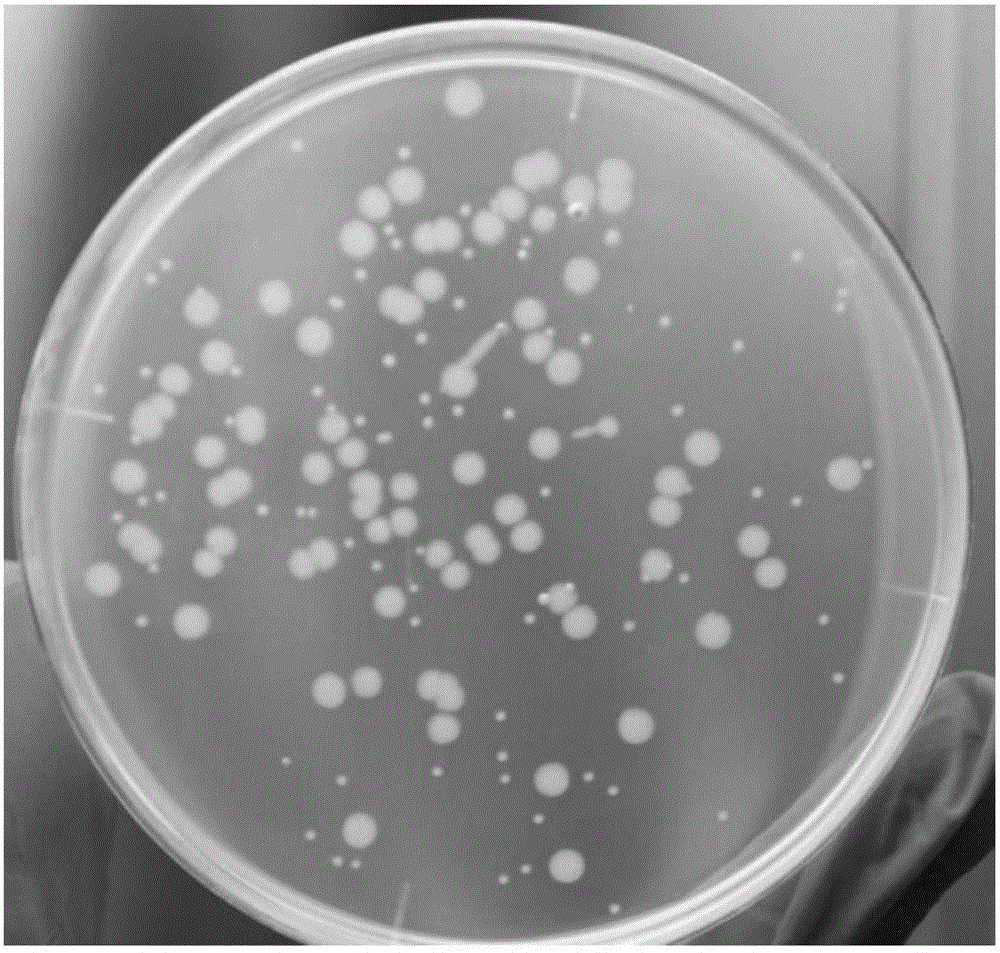
Selective culture medium for detecting antigen bacterium content of swine fever/swine erysipelas/swine plague triple live vaccine, and preparation method and application thereof
A swine pneumonia, selective technology, applied in microorganism-based methods, biochemical equipment and methods, microbial determination/inspection, etc., can solve the problems of swine erysipelas antigen monitoring, etc., to solve the long-term problems of quality monitoring, accurate High precision and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 Accuracy check
[0032] (1) Preparation of agar plates:
[0033] Ordinary agar plate preparation: Weigh 30g TSA powder and dissolve it in 1000mL sterilized double distilled water, pressurize at 121°C for 16min, wait until it cools to 50°C, add 5% bovine serum;
[0034] Preparation of selective agar plate: Weigh 30g TSA powder and dissolve it in 1000mL sterilized double distilled water, pressurize at 121°C for 16min, wait until it cools to 50°C, add 5% bovine serum and 30mg kanamycin.
[0035] (2) Dilute erysipelas G4T10 monovalent live vaccine and porcine pneumonia EO630 monovalent live vaccine with sterile normal saline to 1 mL / head respectively, and take 100 μL and dilute them in 900 μL sterile normal saline respectively, and carry out 6 consecutive times respectively , 100 μL of the sixth dilution was inoculated on a common agar plate without kanamycin, cultured at 37°C for 36 hours, and the colonies were counted.
[0036](3) Mix erysipelas G4T10 monov...
Embodiment 2
[0042] Embodiment 2 repeatability test
[0043] (1) Prepare ordinary agar plates and selective agar plates according to the step (1) of Example 1, dilute the swine fever, swine erysipelas, porcine pneumonia triple vaccine to 1 mL / head, and take 100 μL and dilute it in 900 μL sterile normal saline , carried out 6 times in a row, and respectively took 100 μL of the sixth dilution and inoculated them on ordinary agar plates without kanamycin and selective agar plates containing kanamycin, cultured at 37°C for 36 hours, and normal Count the large colonies and small colonies in the plate respectively, and count all the colonies on the selective agar. The number of large colonies in the ordinary plate is the content of porcine pneumonia antigen, and the number of colonies in the selective plate is the content of porcine erysipelas antigen. Perform 2 repetitions as above.
[0044] (2) compare the results of two repetitions, repeat one: the number of large colonies in the common agar...
Embodiment 3
[0048] Example 3 Detection of different batches of swine fever, swine erysipelas, porcine pneumonia triple live vaccine
[0049] Test group: three different batches of swine fever, swine erysipelas, and porcine pneumonitis triple live vaccine were diluted respectively according to the method of step (1) in the embodiment of the present invention 2, inoculated, cultivated, and then counted, and the number of large colonies in the common plate was As the porcine pneumonia antigen content, the number of colonies in the selective plate is the porcine erysipelas antigen content.
[0050] Control group: Prepare ordinary agar plates and selective agar plates according to the step (1) of Example 1, dilute the swine fever, swine erysipelas, porcine pneumonia triple vaccine to 1 mL / head, and take 100 μL and dilute it in 900 μL sterile saline , carried out 6 times in a row, and respectively took 100 μL of the sixth dilution and inoculated them on ordinary agar plates without kanamycin an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com