Method for measuring hydrolytic enzyme activity
A hydrolytic enzyme and active technology, applied in the field of biological analysis, can solve the problems of complex probe preparation process, insufficient anti-interference ability, poor anti-interference ability, etc., and achieve good reproducibility, short detection time, and strong anti-interference ability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The method of the invention is used in a feasibility analysis experiment for measuring the activity of β-galactosidase.
[0034] 10μL 0.01mg·mL -1 β-galactosidase solution was added to 590 μL containing 10 mM p-aminophenyl-β-D-galactopyranoside, 0.25 mM Fe 3+ and 0.75 mM bathophenanthroline sodium disulfonate in the detection solution, after incubation at 25° C. for 15 min, record the ultraviolet-visible absorption spectrum of the detection solution.
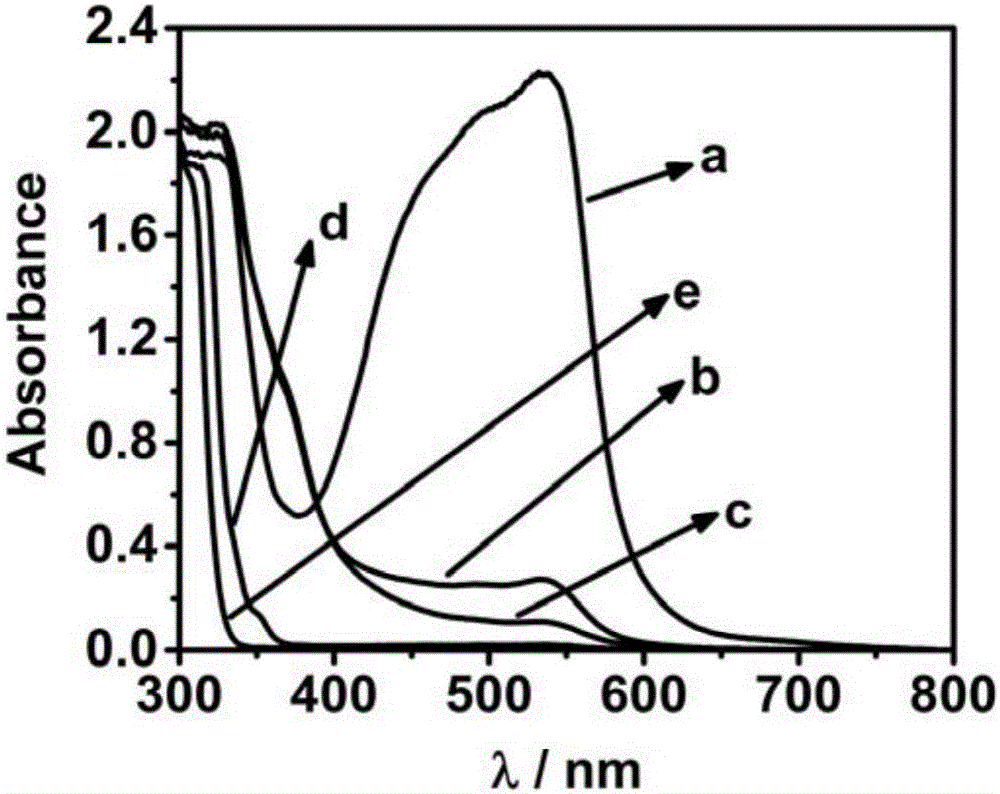
[0035] In feasibility analysis experiments, missing components were replaced with the same volume of buffer. From figure 1 It can be seen that when all components exist (a), the detection solution has a strong absorption peak at ~535nm; and when no β-galactosidase (b), p-aminobenzene Base-β-D-galactopyranoside (c), Fe 3+ (d), or sodium bathophenanthroline disulfonate (e), its absorption value at ~535nm is very low. It can be seen that the method of the present invention can be used to measure the activity of β-galact...
Embodiment 2
[0037] The method of the invention is used for determining the relationship between the detection signal of the β-galactosidase activity and the β-galactosidase activity.
[0038] 10 μL of 0, 20, 40, 60, 80, 100, 120, 140, 160, 180, 200 and 220 mU·mL -1 The β-galactosidase solution was added to 590 μL containing 10 mM p-aminophenyl-β-D-galactopyranoside, 0.25 mM Fe 3+ and 0.75 mM bathophenanthroline sodium disulfonate in the detection solution, after incubation at 25° C. for 15 min, record the ultraviolet-visible absorption spectrum of the detection solution.
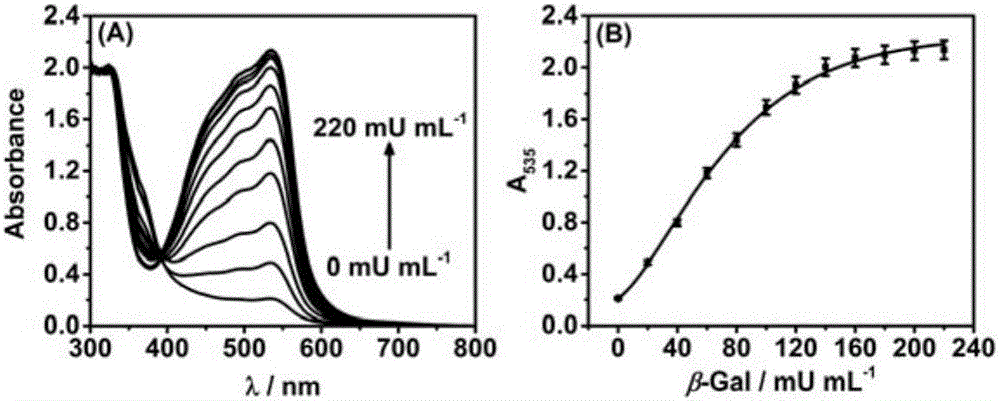
[0039] From figure 2 It can be seen that with the increase of β-galactosidase activity, the absorption value of the detection solution at ~535nm increases correspondingly, and the absorption intensity has a good quantitative relationship with β-galactosidase activity. It can be seen that the method of the present invention can be used for quantitative determination of β-galactosidase activity.
Embodiment 3
[0041] The method of the invention is used to determine the selectivity of β-galactosidase activity.
[0042] 10μL 0.01mg·mL -1 β-galactosidase or 10μL 0.1mg·mL -1 The other protein solution was added to 590 μL containing 10 mM p-aminophenyl-β-D-galactopyranoside, 0.25 mM Fe 3+ and 0.75 mM bathophenanthroline sodium disulfonate in the detection solution, after incubation at 25° C. for 15 min, record the absorption value of the detection solution at ~535 nm.
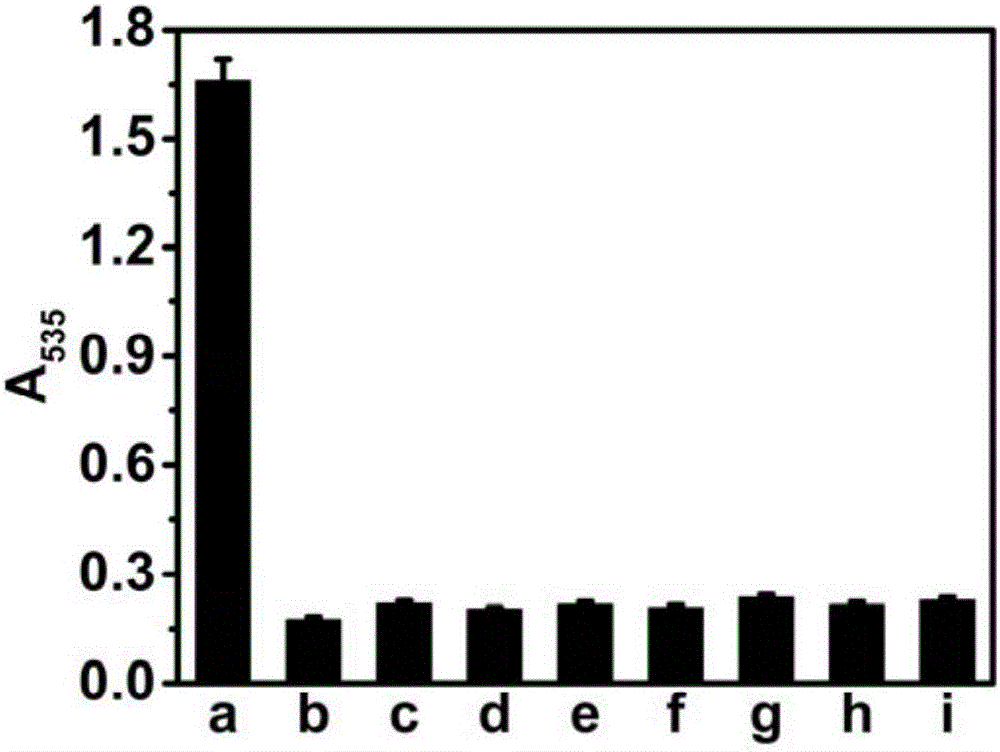
[0043] From image 3 It can be seen that only when β-galactosidase is added, the absorption value of the detection solution at ~535nm will increase significantly. It can be seen that the method of the present invention has high selectivity when used for measuring the activity of β-galactosidase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com