Nat10 modulators for treating or preventing laminopathies, aging and cancer
A lamin, cancer technology, applied in the field of NAT10 modulators for the treatment or prevention of laminopathy, aging and cancer, capable of solving problems such as molecular causes to be determined
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0138] Compound preparation
[0139] Herein, the term "pharmaceutically acceptable salt" is intended to describe a salt that is not harmful to the patient. Such salts include pharmaceutically acceptable acid addition salts, pharmaceutically acceptable metal salts and pharmaceutically acceptable base addition salts. Acid addition salts include salts of inorganic acids as well as organic acids.
[0140] References to compounds of formula (I) and groups thereof also include, for example, ionic forms, salts, solvates, isomers (including geometric and stereochemical isomers), tautomers, N-oxides as described below , esters, prodrugs, isotopes and protected forms thereof; preferably salts or tautomers or isomers or N-oxides or solvates thereof; more preferably salts or tautomers or N- Oxides or solvates, even more preferably salts or tautomers or solvates thereof. Hereinafter, compounds defined in any aspect of the invention and their ionic forms, salts, solvates, isomers (incl...
Embodiment 1
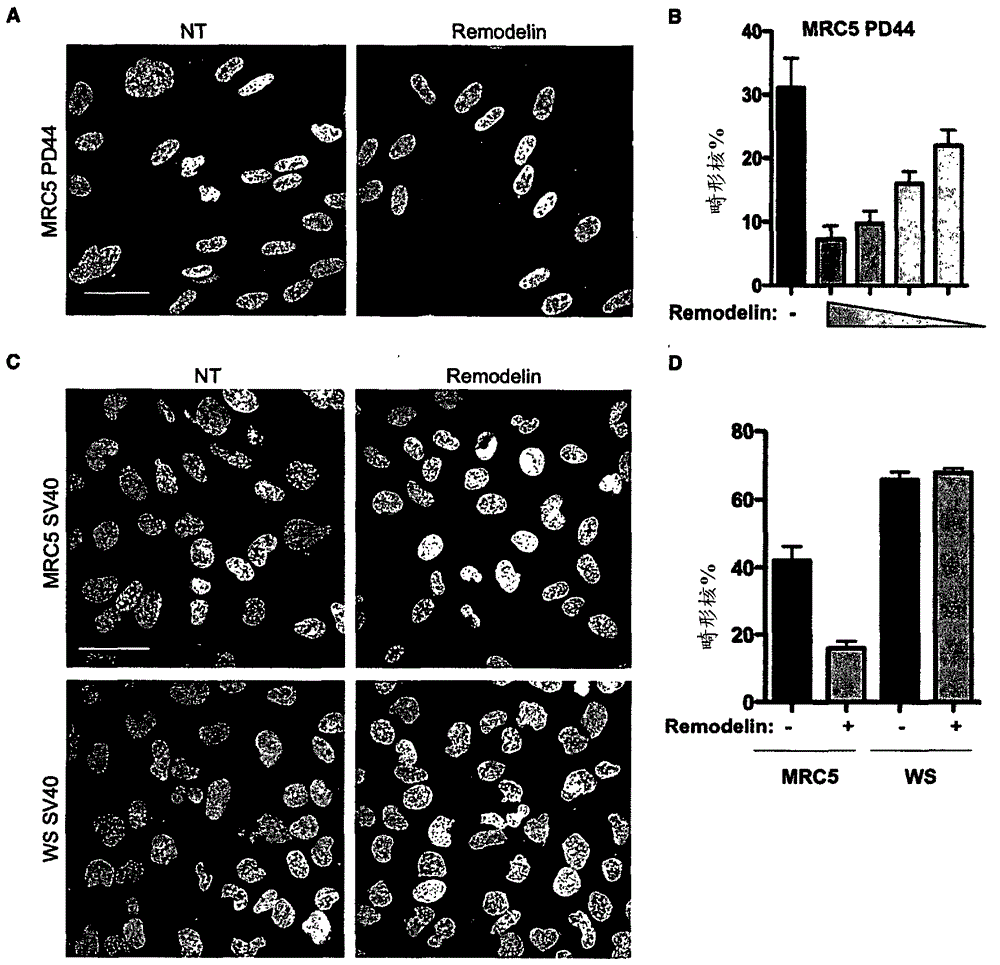
[0330] Example 1: Rescue of nuclear shape by KAT inhibitors
[0331] Because the lamin protein acts as a scaffold to maintain nuclear structure and anchor chromatin at the nuclear edge (J.Gotzmann, R. Foisner (1999) Crit. Rev. Eukaryot. Gene Expr. 9, 257), so small interfering RNA (siRNA)-mediated depletion of lamin A / C (siLMNA) caused nuclear shape defects in different human cell lines ( figure 1 A, B and figure 2 A, B). Notably, lamin A / C depletion was found to be also associated with overall chromatin relaxation, as observed by enhanced micrococcal nuclease (MNase) sensitivity and increased nuclear area ( figure 1 B, C). Thus, it was reasoned that recompaction of chromatin in lamin A / C depleted cells could ameliorate some of their nuclear structural defects. Lysine acetyltransferase (KAT) and lysine deacetylase (KDAC) have the ability to regulate the acetylation status of histones and other proteins, thus affecting the overall chromatin organization. Therefore, th...
Embodiment 2
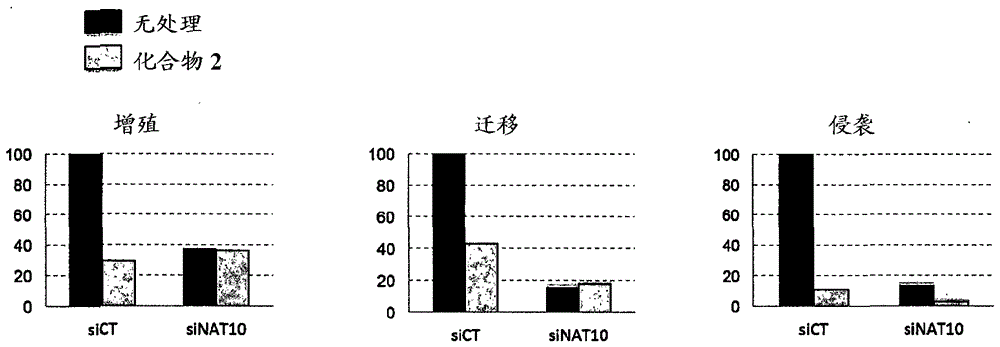
[0332] Example 2: Identification of Protein Targets by Chemical Labeling of Compound 2
[0333] To identify putative biological targets of molecular compound 2 and elucidate the mechanism for improved nuclear morphology, a molecular-based strategy was established that included cellular functionalization of the molecule by means of "click chemistry" to find and validate drug-associated protein complexes. To this end, an alkynyl group was strategically introduced into the hydrazone moiety of compound 2, resulting in a Figure 4 B) "clickable" analog compound 3 with equivalent cellular activity ( Figure 4 A), and use an inactive clickable molecule reference compound A as a negative control ( Figure 4 A, B). Although the alkyne moiety is biologically inert, it can react selectively with any molecule bearing an azide group upon copper exposure (V. V. Rostovtsev et al. (2002) Angew. Chem. Int. Ed. Engl. 41, 2596 ), thus allowing the labeling of small molecules in cells ( Fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com