Preparation method of lithium difluorophosphate
A technology of lithium difluorophosphate and lithium hexafluorophosphate, which is applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve problems such as unfavorable safety production, energy saving and emission reduction, high requirements for reaction conditions, inconvenient control, etc., and is easy to realize , high yield, cost-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
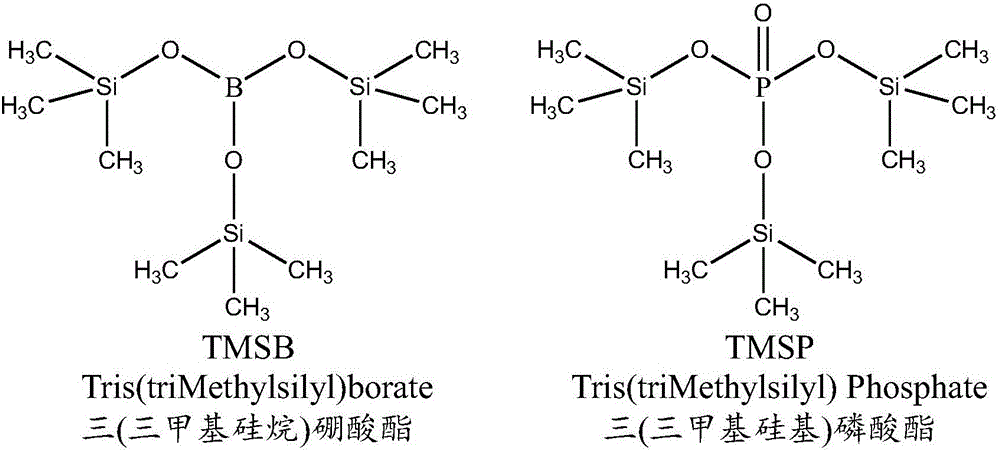
[0027] Set up the reaction device, first pass dry nitrogen to replace the air, stop nitrogen, immediately put 45.57g lithium hexafluorophosphate (about 0.3mol) into a 500mL four-neck round bottom flask, add 227.85g ethyl acetate to dissolve lithium hexafluorophosphate, turn on nitrogen and Heat and stir to raise the temperature of the solution to 40°C, and then add 55.67 g of TMSB (about 0.2 mol) dropwise. During the dropwise addition, bubbles appear in the solution and solids are precipitated. The dropwise addition process takes 50 minutes. After the dropwise addition, the temperature was slowly raised to 65°C, and the bubbles in the solution contained in the four-neck round-bottomed flask increased significantly, so the nitrogen flow rate was reduced to stabilize the gas flow rate in the tail gas absorption device. With the gradual progress of the reaction, the amount of bubbles observed in the four-necked round-bottom flask decreased, and the nitrogen flow was increased. At ...
Embodiment 2
[0030] Set up the reaction device, first feed dry nitrogen to replace the air, stop feeding nitrogen, immediately drop into 45.57g lithium hexafluorophosphate (about 0.3mol) into the 500mL four-neck round bottom flask, then add 200g of ethyl acetate recovered in Example 1, Turn on the nitrogen gas, and slowly add 61.24g TMSB (about 0.22mol) dropwise after the solution is heated to 35°C. Bubbles appear in the solution and solids are precipitated during the dropwise addition process. The dropwise addition process takes 40 minutes. Continue to heat up to 70°C, the bubbles in the solution will increase significantly, and the solid matter precipitated in the solution will also increase significantly. Adjust the flow of nitrogen to stabilize the flow of gas in the tail gas absorption device. After reacting for a certain period of time, the amount of bubbles that can be observed in the four-necked round-bottom flask decreases, and the nitrogen flow is increased to prevent the tail gas...
Embodiment 3
[0033] Set up the reaction device, first replace the air with dry nitrogen, stop nitrogen, immediately put 50.13g of lithium hexafluorophosphate (about 0.33mol) into a 500mL four-neck round bottom flask and add 227.85g of 1,4-dioxane to dissolve and put in Lithium hexafluorophosphate, turn on nitrogen, heat to keep the solution at 30°C, at this time there is still a small amount of lithium hexafluorophosphate undissolved, slowly add 62.91g TMSP (about 0.2mol) dropwise, bubbles gradually appear in the solution. After 60 minutes, the dropwise addition of TMSP was completed, and the temperature of the solution was slowly raised to 60°C. The bubbles in the solution gradually increased, and the precipitated solids in the flask also increased significantly. Reduce the nitrogen flow rate to stabilize the gas flow rate in the tail gas absorption device. After reacting for a period of time, the amount of bubbles observed in the flask decreases, and the nitrogen flow is increased to avoi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com