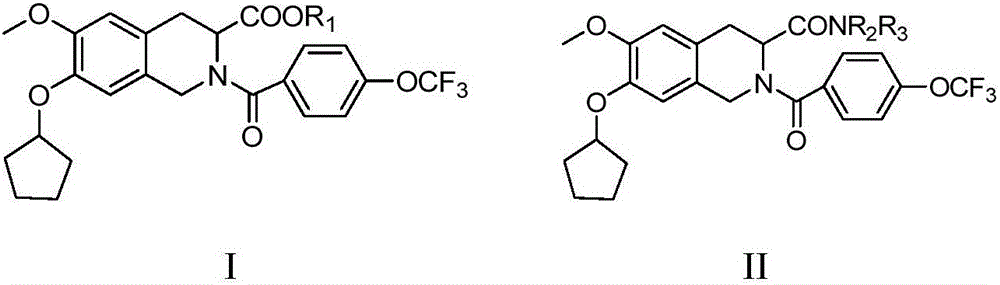
C3-substituted tetrahydroisoquinoline derivative and preparation method and application thereof
A technology of tetrahydroisoquinoline and derivatives, which can be used in drug combinations, blood diseases, extracellular fluid diseases, etc., can solve problems such as treatment cost burden, and achieve the effects of good biological activity, novel structure and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
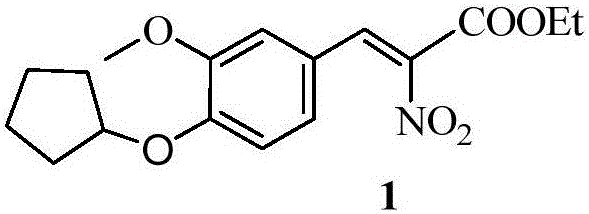
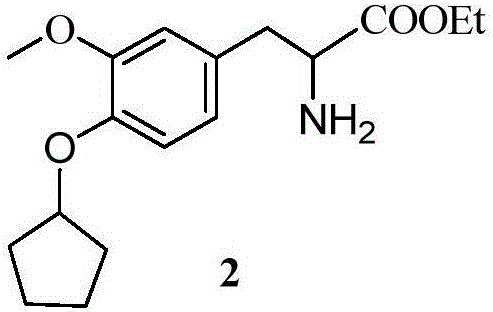
[0066] Example 1: 7-cyclopentyloxy-8-methoxy-1,5,10,10a-tetrahydro-2-p-trifluoromethoxybenzoyl[3,4-b]isoquinoline- Preparation of ethyl 3-formate (5)
[0067] Using vanillin as a starting material, undergo substitution, addition elimination, reduction, acylation, Bischler-Napieralski reaction and acylation reaction to obtain tetrahydroisoquinoline and carbamate compounds. The preparation process is as follows:
[0068]
[0069] Reagents and conditions: a.K 2 CO 3 ,DMF; b.NO 2 CH 2 COOEt, (CH 3 ) 2 NH·HCl, KF; c.H 2 ,10%Pd / C; d.HCOOH,EDC,DMAP; e.I.POCl 3 , toluene; II.H 2 , 10% Pd / C, MeOH, EtOAc; f.dry CH 2 Cl 2 ,Et 3 N, DMAP; g MeONa / MeOH; h 4N NaOH, THF / MeOH; I K 2 CO 3 , n-PentylBr, DMF; j EDC-HCl, DMAP, CH 2 Cl 2
[0070] (1) Preparation of 4-cyclopentyloxy-3-methoxybenzaldehyde (I-A)
[0071] Dissolve (25g, 0.164mol) vanillin in 80mL DMF, add (33g, 0.239mol) potassium carbonate solid, stir at room temperature for half an hour, then add (22mL, 0.193mol)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com