Fast high-selectivity super-sensitive nickel ion colorimetric fluorescent probe and preparation method thereof
A nickel ion, fluorophore technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of complex synthesis, insufficient sensitivity, and insufficient response speed, and achieve simple synthesis, good stability, Responsive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] (Scheme 1) Dissolve 410.5mg (1mmol) of rhodamine-like fluorophore compound in 15mL of anhydrous dichloromethane, then add 120.5mg (1mmol) of allyl chloroformate, then stir and react at 25°C for 3h, after the reaction The dichloromethane was evaporated to dryness to obtain the crude product. Finally, a mixed system of dichloromethane and petroleum ether (1:5, v / v) was used for column chromatography to obtain 306.6 mg of a pink pure product with a yield of 62%.
[0035] (Scheme 2) Dissolve 410.5mg (1mmol) of rhodamine-like fluorophore compound in 15mL of anhydrous dichloromethane, then add 180.8mg (1.5mmol) of allyl chloroformate, then stir and react at 25°C for 3h, and the reaction ends Afterwards, the dichloromethane was evaporated to dryness to obtain the crude product. Finally, a mixed system of dichloromethane and petroleum ether (1:5, v / v) was used for column chromatography to obtain 385.7 mg of a pink pure product with a yield of 78%.
[0036] (Scheme...
Embodiment 2
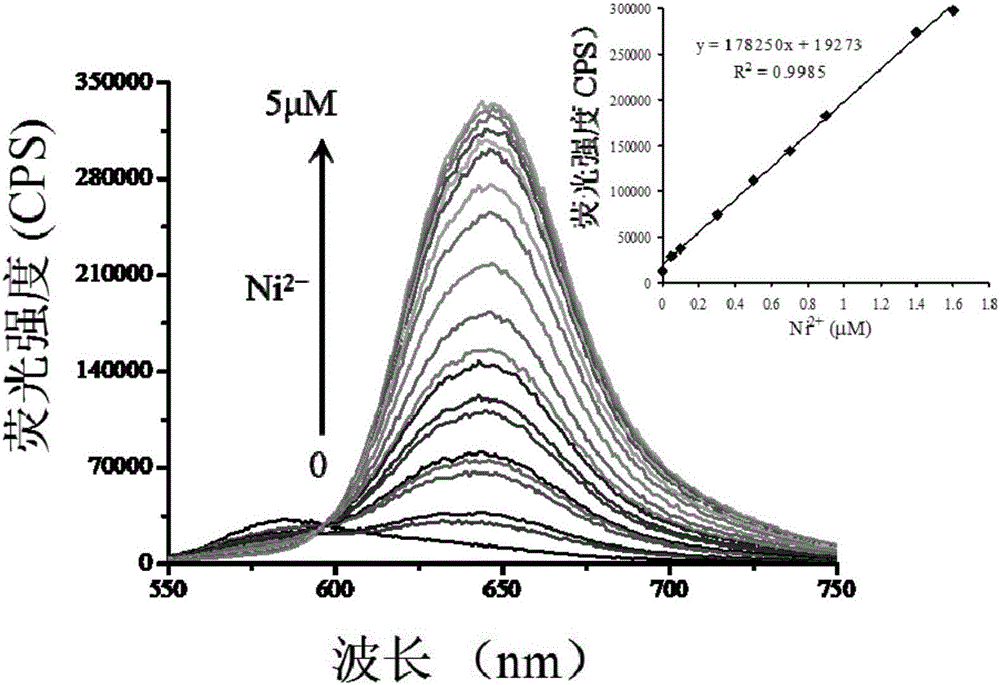
[0040] Fig. 1(a) is a diagram of the change in fluorescence spectrum of the nickel ion colorimetric fluorescent probe (20 μM) after adding nickel ions (0-5 μM). It can be clearly seen from the figure that with the increase of the concentration of nickel ions added, the fluorescence intensity at 645 nm of the solution gradually increases. Moreover, it can be seen from the illustration that the fluorescence intensity at 645 nm has a good linear relationship with the concentration of the added nickel ions, which proves that the fluorescent probe can be used for quantitative analysis of nickel ions.
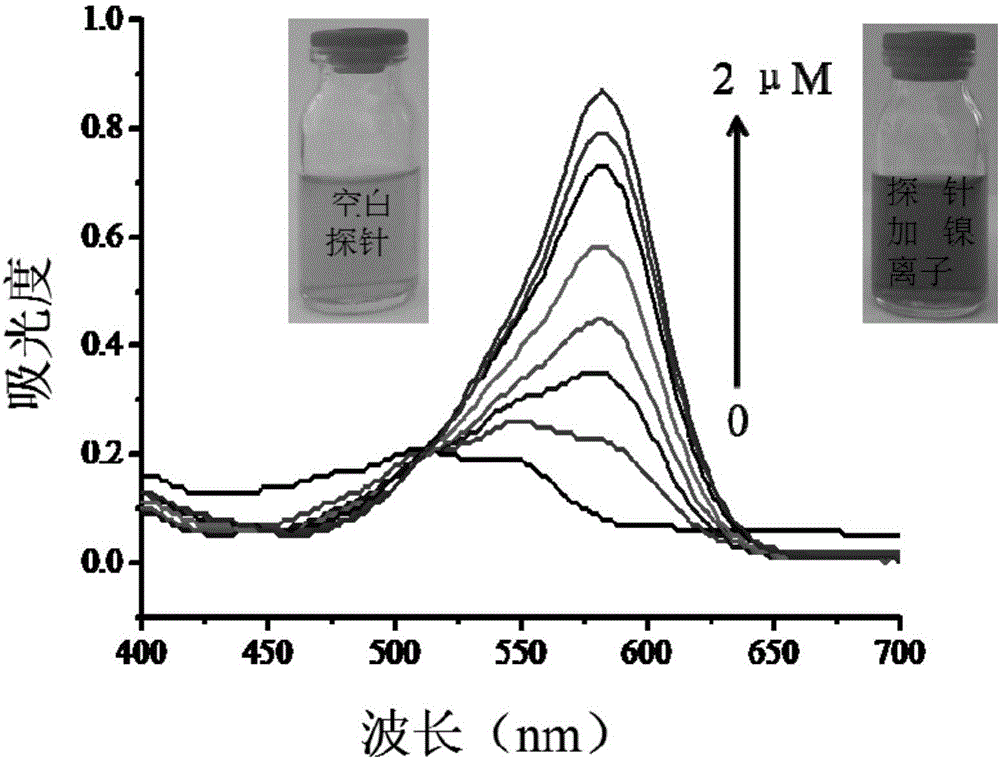
[0041] Fig. 1(b) is a change diagram of the absorption spectrum of the nickel ion colorimetric fluorescent probe (20 μM) after adding nickel ions (0-2 μM). It can be seen from the figure that as the concentration of nickel ions increases, the absorbance value at 580 nm of the solution increases gradually. From the illustration, we can clearly see that after adding nickel ions, the c...
Embodiment 3
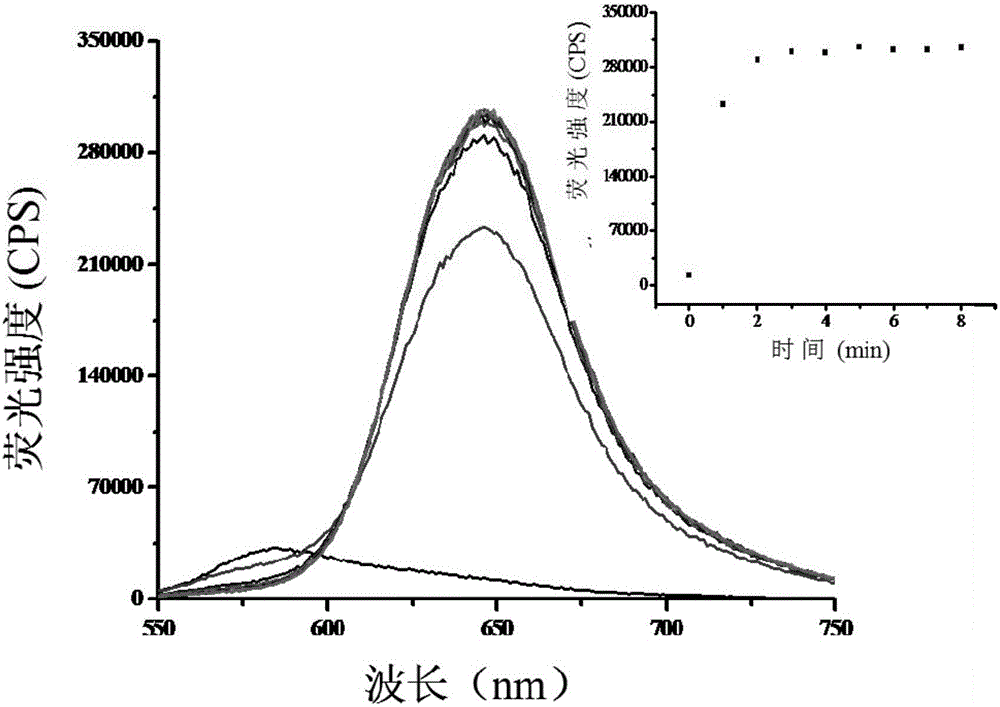
[0043] figure 2 It is a graph showing the change of fluorescence spectrum with time after adding nickel ions (2 μM) to the nickel ion colorimetric fluorescent probe (20 μM). It can be clearly seen from the figure that after the addition of nickel ions, the fluorescence intensity reaches the maximum after 2 minutes and remains unchanged, which indicates that the probe reacts rapidly with nickel ions and can provide a rapid analysis method for the determination of nickel ions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com