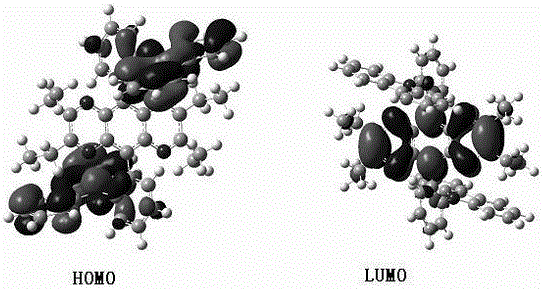
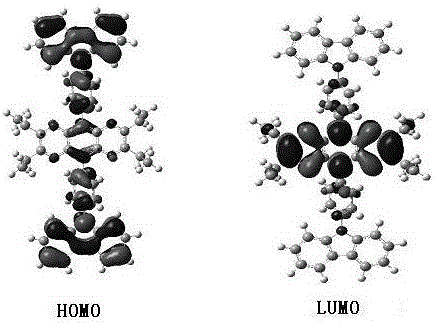
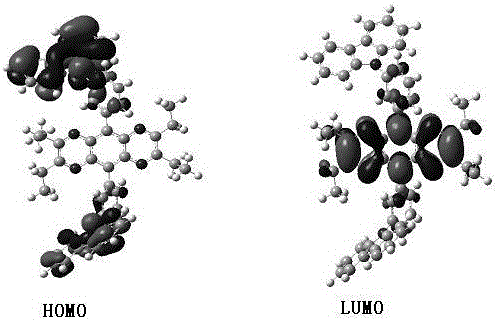Ethylize-pyrazine and quinoxaline derivative and preparation method thereof
A technology of ethylated pyrazine and derivatives, which is applied in the fields of chemical instruments and methods, organic chemistry, semiconductor/solid-state device manufacturing, etc., can solve the problems of limited development, exciton annihilation, expensive phosphorescent materials, etc., and achieve high injection efficiency. Efficiency, the effect of lowering the injection barrier
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment one: the synthesis of compound M1
[0042]
[0043] Add trifluoromethanesulfonic acid (15g, 8.85mL, 100mmol) to a 100mL two-necked flask, add fuming nitric acid (1.5g, 0.99mL, 35.7mmol) dropwise under ice bath, and then transfer the two-necked flask to 50°C in an oil bath. Add 4,7-dibromo-2,1,3-benzothiadiazole (2.500g, 8.5mmol) into the two-necked flask, and stir the reaction overnight. After the reaction, the reaction liquid was poured into 200 mL of ice-water mixture, the pH of the solution was adjusted to 7, suction filtered and dried, and the crude product was recrystallized with absolute ethanol to obtain 1.746 g of light yellow crystals, with a yield of 53.4%. MS: m / z=381.8018 [M] +
[0044] Weigh 4,7-dibromo-5,6-dinitro-2,1,3-benzothiadiazole (1.000g, 2.621mmol), add reduced zinc powder (3.406g, 52.4mmol) to 100mL dry di Add 20mL glacial acetic acid as a solvent to the flask, react in an oil bath at 60°C for 1.5h under nitrogen protection, add ...
Embodiment 2
[0046] Embodiment two: the synthesis of compound M2
[0047]
[0048] Add trifluoromethanesulfonic acid (15g, 8.85mL, 100mmol) to a 100mL two-necked flask, add fuming nitric acid (1.5g, 0.99mL, 35.7mmol) dropwise under ice bath, and then transfer the two-necked flask to 50°C in an oil bath. Add 4,7-dibromo-2,1,3-benzothiadiazole (2.500g, 8.5mmol) into the two-necked flask, and stir the reaction overnight. After the reaction, the reaction liquid was poured into 200 mL of ice-water mixture, the pH of the solution was adjusted to 7, suction filtered and dried, and the crude product was recrystallized with absolute ethanol to obtain 1.746 g of light yellow crystals, with a yield of 53.4%. MS: m / z=381.8018 [M] +
[0049] Weigh 4,7-dibromo-5,6-dinitro-2,1,3-benzothiadiazole (1.000g, 2.621mmol), add reduced zinc powder (3.406g, 52.4mmol) to 100mL dry di Add 20mL glacial acetic acid as a solvent to the flask, react in an oil bath at 60°C for 1.5h under nitrogen protection, add ...
Embodiment 3
[0051] Embodiment three: the synthesis of compound M3
[0052]
[0053] Add trifluoromethanesulfonic acid (15g, 8.85mL, 100mmol) to a 100mL two-necked flask, add fuming nitric acid (1.5g, 0.99mL, 35.7mmol) dropwise under ice bath, and then transfer the two-necked flask to 50°C in an oil bath. Add 4,7-dibromo-2,1,3-benzothiadiazole (2.500g, 8.5mmol) into the two-necked flask, and stir the reaction overnight. After the reaction, the reaction liquid was poured into 200 mL of ice-water mixture, the pH of the solution was adjusted to 7, suction filtered and dried, and the crude product was recrystallized with absolute ethanol to obtain 1.746 g of light yellow crystals, with a yield of 53.4%. MS: m / z=381.8018 [M] +
[0054] Weigh 4,7-dibromo-5,6-dinitro-2,1,3-benzothiadiazole (1.000g, 2.621mmol), add reduced zinc powder (3.406g, 52.4mmol) to 100mL dry di Add 20mL glacial acetic acid as a solvent to the flask, react in an oil bath at 60°C for 1.5h under nitrogen protection, ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com