Bridged bis-benzimidazole salt as well as preparation method and application thereof
A kind of bisbenzimidazole and reaction technology, applied in the field of bridged bisbenzimidazole salt preparation, and can solve the problems such as wide application to be developed and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
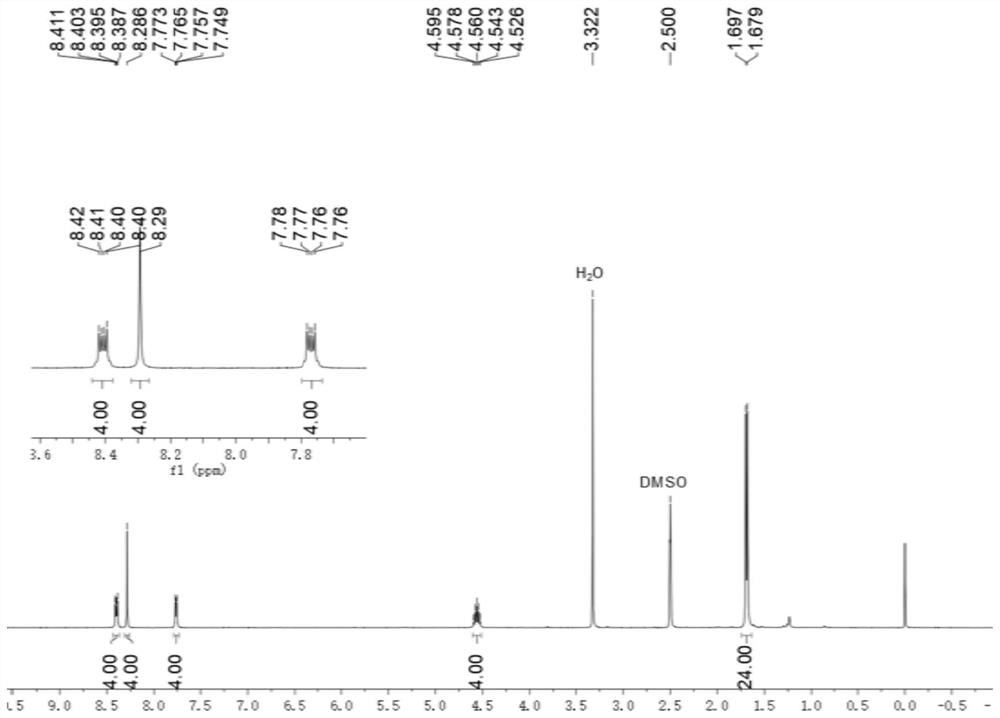
[0073] to N 1 ,N 2 To a solution of diisopropylbenzene-1,2-diamine (768 mg, 4.00 mmol) in dichloromethane (3 mL) was added terephthalaldehyde (1 mmol, 134 mg). Then 0.35 mL of glacial acetic acid was added, and the resulting mixed solution was stirred at 50 °C at a rate of 100 rpm for 16 hours, and the solvent was removed in vacuo under reduced pressure to obtain 1,4-bis(1,3-diisopropyl-2, Crude product of 3-dihydro-1H-benzimidazol)-2-yl)benzene.
[0074] To a solution of crude 1,4-bis(1,3-diisopropyl-2,3-dihydro-1H-benzimidazol-2-yl)benzene in tetrahydrofuran (6 mL) was added 2-bromoacetophenone (2.1 mmol, 418 mg) and stirred at room temperature for 20 hours. The filtrate was removed and the residue was purified by silica gel chromatography (methanol / dichloromethane volume ratio = 1:10) to give Ar ( i Pr)A 1 ·2Br - , (0.7 mmol, 334 mg).
[0075] Ar ( i Pr)A 1 ·2Br - and methyl trifluoromethanesulfonate (0.95 mmol, 156 mg) were mixed in dry dichloromethane (3 mL), an...
Embodiment 2
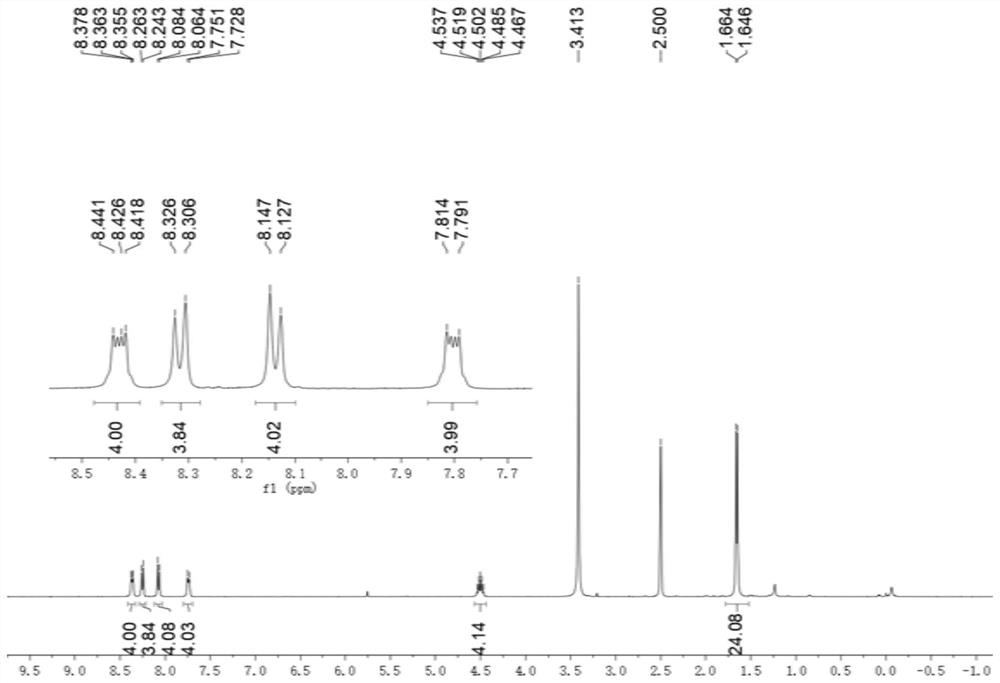
[0082] to N 1 ,N 2 -Diisopropylbenzene-1,2-diamine (768 mg, 4.00 mmol) in dichloromethane (3 mL) was added biphenyldicarbaldehyde (1 mmol, 210.2 mg). Then 0.35 mL of glacial acetic acid was added, and the resulting mixed solution was stirred at 50° C. at a rate of 100 rpm for 16 hours, and the solvent was removed under reduced pressure and vacuum to obtain 4,4′-bis(1,3-diisopropyl-2 ,3-dihydro-1H-benzimidazol-2-yl)-1,1'-biphenyl crude product.
[0083] To a solution of crude 4,4'-bis(1,3-diisopropyl-2,3-dihydro-1H-benzimidazol-2-yl)-1,1'-biphenyl in tetrahydrofuran (6 mL ) was added with 2-bromoacetophenone (2.1 mmol, 418 mg), followed by stirring at room temperature for 20 hours. The filtrate was removed and the residue was purified by silica gel chromatography (methanol / dichloromethane volume ratio = 1:10) to give Ar ( i Pr)A 2 ·2Br - (0.7 mmol, 502.3 mg).
[0084] Ar ( i Pr)A 2 ·2Br - and methyl trifluoromethanesulfonate (0.95 mmol, 156 mg) were mixed in dry dichl...
Embodiment 3
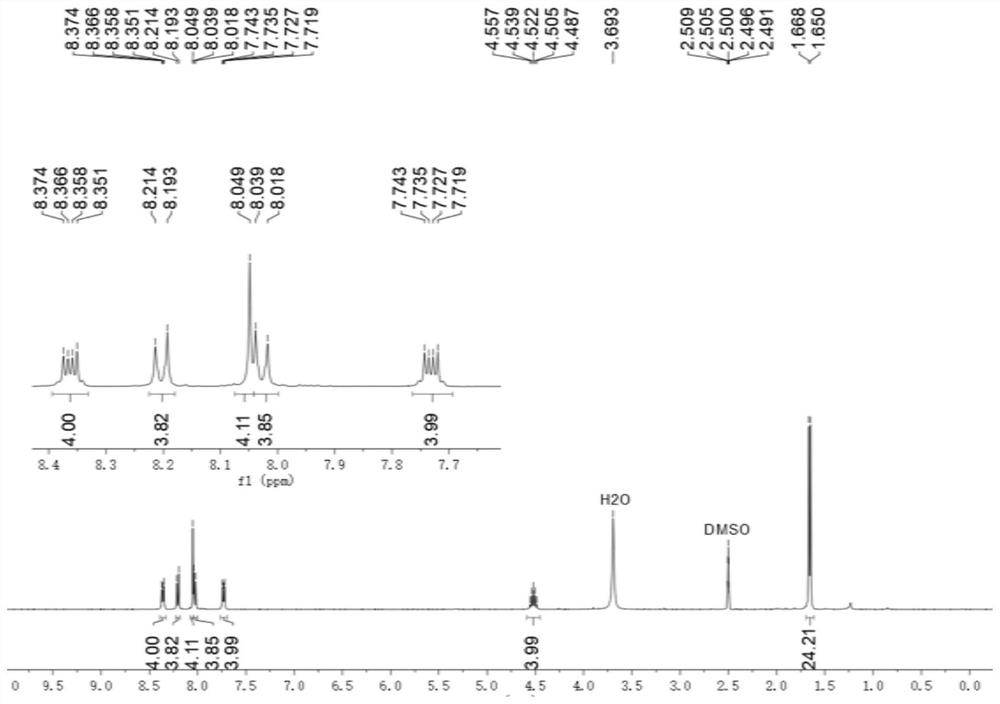
[0091] to N 1 ,N 2 - Diisopropylbenzene - 1,2-diamine (768 mg, 4.00 mmol) in dichloromethane (3 mL) was added terphenyl - 4,4" - dicarbaldehyde (1 mmol, 286.3 mg). Then 0.35 mL was added Glacial acetic acid, the resulting mixed solution was stirred at 50 °C at a rate of 100 rpm, reacted for 16 hours, and the solvent was removed under reduced pressure to obtain 4,4"-bis(1,3-diisopropyl-2,3-diisopropyl) Hydrogen-1H-benzimidazol-2-yl)-1,1':4',1"-crude product of terphenyl.
[0092] To 4,4"-bis(1,3-diisopropyl-2,3-dihydro-1H-benzimidazol-2-yl)-1,1':4',1"-terphenyl crude product 2-Bromoacetophenone (2.1 mmol, 418 mg) was added to the tetrahydrofuran solution (6 mL), and the mixture was stirred at room temperature for 20 hours. The filtrate was removed and the residue was purified by silica gel chromatography (methanol / dichloromethane volume ratio = 1:10) to give Ar ( i Pr)A 3 ·2Br - (0.7 mmol, 554.9 mg).
[0093] Ar ( i Pr)A 3 ·2Br - and methyl trifluoromethanesulfonate (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com