Complex phosphorescent materials based on electron-deficient functional groups
A technology of functional groups and phosphorescent light-emitting materials, applied in the field of complex phosphorescent materials, can solve the problem of not paying much attention to the excited state of higher energy level, and achieve optimized carrier injection and transport properties, excellent display effect, and enhanced electroluminescence. effect of ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
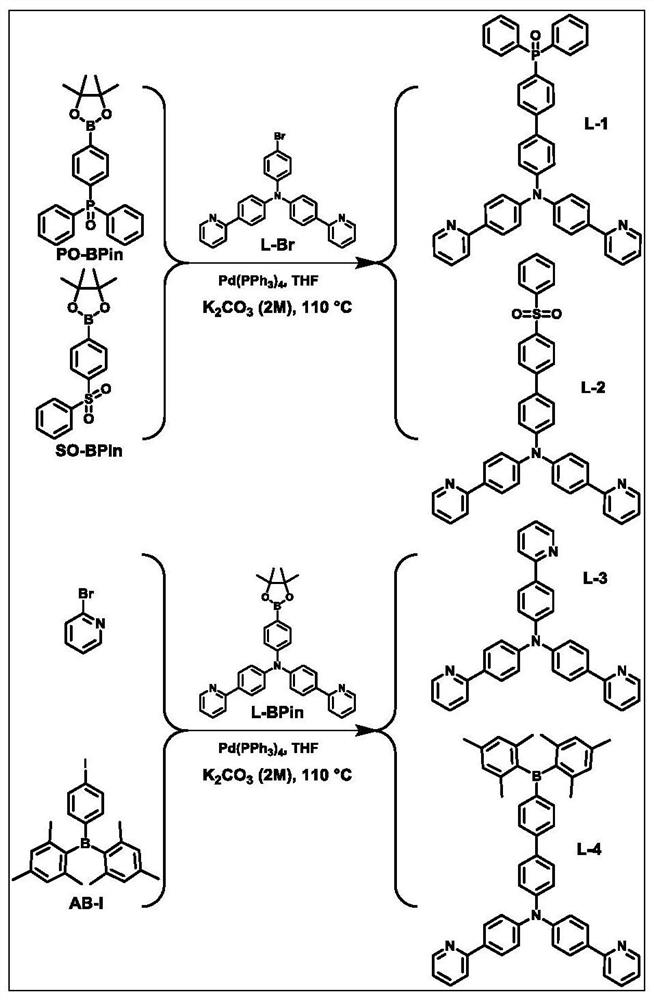
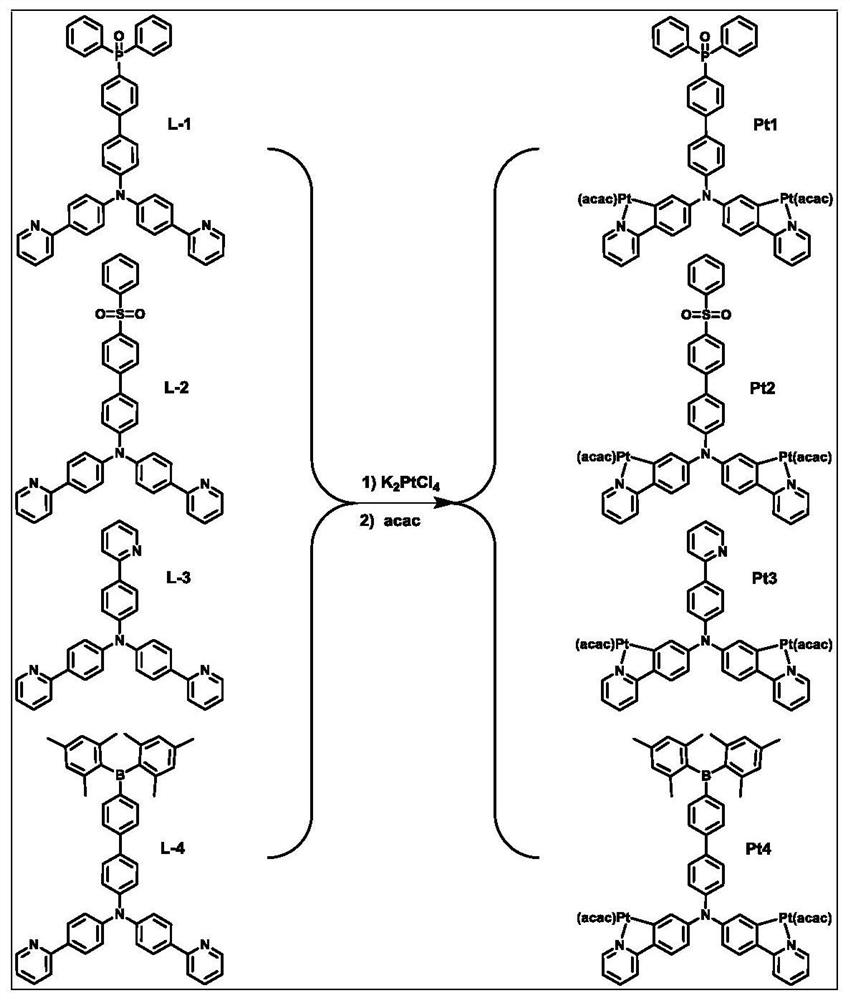
[0034] The structural formula of the ligand L-1 based on the electron-deficient functional group in this embodiment is: refer to figure 1 , the synthesis method is as follows: 1 equivalent of electron-deficient functional group compound and 1 equivalent four-tooth precursor Drop into the reaction vessel, under nitrogen atmosphere, add 0.05% equivalent of tetrakis (triphenylphosphine palladium) catalyst, 50 ml of tetrahydrofuran and 30 ml of potassium carbonate solution with a concentration of 2mol / L to the reaction vessel, in a nitrogen atmosphere The reaction was heated to 110° C. overnight; the heating was stopped, and after cooling to room temperature, deionized water was added to the reaction mixture, and the reaction mixture was extracted with dichloromethane. The obtained organic phase is dried with anhydrous sodium sulfate, concentrated, separated and purified by silica gel column, and then the corresponding ligand L-1 based on the electron-deficient functional gro...
Embodiment 2
[0037] The structural formula of the ligand L-2 based on the electron-deficient functional group in this embodiment is: refer to figure 1 , the synthesis method is as follows: 1 equivalent of electron-deficient functional group compound and 1 equivalent four-tooth precursor Drop into the reaction vessel, under nitrogen atmosphere, add 0.05% equivalent of tetrakis(triphenylphosphine palladium) catalyst, 50 ml of tetrahydrofuran and 30 ml of potassium carbonate solution with concentration of 2mol / L into the reaction vessel, in nitrogen atmosphere Heating to 110°C for overnight reaction; stopping heating, cooling to room temperature, adding deionized water to the reaction mixture, extracting the reaction mixture with dichloromethane; drying the obtained organic phase with anhydrous sodium sulfate, concentrating, and separating and purifying with silica gel column, namely The corresponding ligand L-2 based on electron-deficient functional groups can be obtained. The NMR char...
Embodiment 3
[0040] The structural formula of the ligand L-3 based on the electron-deficient functional group in this embodiment is: refer to figure 1 , the synthesis method is as follows: 1 equivalent of electron-deficient functional group compound and 1 equivalent four-tooth precursor Drop into the reaction vessel, under nitrogen atmosphere, add 0.05% equivalent of tetrakis (triphenylphosphine palladium) catalyst, 50 ml of tetrahydrofuran and 30 ml of potassium carbonate solution with a concentration of 2mol / L to the reaction vessel, in a nitrogen atmosphere Heating to 110 °C and reacting overnight; stopping heating, cooling to room temperature, adding deionized water to the reaction mixture, and extracting the reaction mixture with dichloromethane; drying the obtained organic phase with anhydrous sodium sulfate, concentrating, and separating and purifying with a silica gel column, namely The corresponding ligand L-3 based on electron-deficient functional groups can be obtained. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com