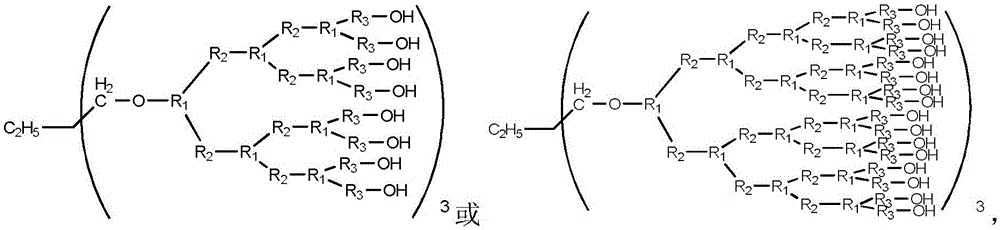
Phosphorus-nitrogen containing halogen-free flame-retardant hyperbranched epoxy resin and preparation method thereof
An epoxy resin, flame retardant technology, which is applied in the field of phosphorus-nitrogen-free halogen-free flame retardant hyperbranched epoxy resin and its preparation, can solve the problem of no flame retardant function and the like, and achieve good catalytic activity and selectivity , The effect of low raw material cost and low thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Mix 216g of 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide DOPO, 112g of itaconic anhydride ITA and 184g of toluene, stir and react at 120°C for 4h, filter after the reaction, 60 ℃ vacuum drying for 12h to obtain DOPO-ITA. 164.0g DOPO-ITA, 66.5g diisopropanolamine DIPA, 22.3g trimethylolpropane, 0.32g 1-butylsulfonic acid-3-methylimidazolium bisulfate ([BHSO 3 MIM]HSO 4 ) and 0.32g of 1-ethyl-3-methylimidazolium bisulfate (EMIMHSO 4 ) at 180° C., heated and mixed evenly, and stirred for 1 h to obtain the first-generation hydroxyl-terminated hyperbranched polyester amide HBPP-1. 73.2g HBPP-1, 35.0g mercaptopropionic acid, 46.0g toluene and 0.9g p-toluenesulfonic acid were reacted at 80°C for 10h. After the reaction, the toluene and excess mercaptopropionic acid were removed under reduced pressure to obtain the mercapto-terminated hyperbranched polymer TBPP- 1. Mix 40.7g TBPP-1, 17.1g allyl glycidyl ether AGE and 0.1g 1-hydroxycyclohexyl phenyl ketone evenly, stir...
Embodiment 2
[0033] Mix 216.0g of 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide DOPO, 112.0g of itaconic anhydride ITA, 106.0g of xylene and 72.0g of tetrahydrofuran, and stir the reaction at 100°C After 6 hours of reaction, filter and dry at 60° C. for 12 hours to obtain 263.9 g of DOPO-ITA. 164.0g DOPO-ITA, 66.5g diisopropanolamine DIPA, 7.4g trimethylolpropane, 0.35g 1-butylsulfonic acid-3-methylimidazolium bisulfate ([BHSO 3 MIM]HSO 4 ) and 0.35g of 1-ethyl-3-methylimidazolium bisulfate (EMIMHSO 4) at 170° C., heated and mixed evenly, and stirred for 2 hours to obtain the second-generation hydroxyl-terminated hyperbranched polyester amide HBPP-2. 82.4g of HBPP-2, 27.9g of mercaptopropionic acid, 44.2g of toluene and 1.0g of p-toluenesulfonic acid were reacted at 100°C for 9 hours. After the reaction, the toluene and mercaptopropionic acid were removed under reduced pressure to obtain TBPP-2, a mercapto-terminated hyperbranched polymer. Mix 45.5g TBPP-2, 20.6g AGE and 0.2g 2,4,6...
Embodiment 3
[0035] Mix 216.0g of 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide DOPO, 112.0g of itaconic anhydride ITA and 212.0g of xylene, stir and react at 80°C for 8h, and the reaction ends Then filter and dry to obtain 266.5g DOPO-ITA. 164.0g DOPO-ITA, 66.5g diisopropanolamine DIPA, 3.2g trimethylolpropane, 0.45g 1-butylsulfonic acid-3-methylimidazolium bisulfate ([BHSO 3 MIM]HSO 4 ) and 0.45g 1-ethyl-3-methylimidazolium bisulfate (EMIMHSO 4 ) at 160° C., heated and mixed evenly, and stirred for 3 hours to obtain the third-generation hydroxyl-terminated hyperbranched polyester amide HBPP-3. 94.4g of HBPP-3, 27.9g of mercaptopropionic acid, 92.0g of toluene and 1.1g of p-toluenesulfonic acid were reacted at 90°C for 8 hours. After the reaction, the toluene and mercaptopropionic acid were removed under reduced pressure to obtain TBPP-3, a mercapto-terminated hyperbranched polymer. Mix 103.0g TBPP-3, 41.1g allyl glycidyl ether AGE and 0.5g 2-methyl-1-[4-methylthiophenyl]-2-morpho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com