Recombinant retrovirus for primary T cell infection, infection method and application thereof
A retrovirus and cell infection technology, applied in the field of recombinant retroviruses for primary T cell infection, can solve the problems of low feasibility, no means of delivery, hindered development, etc., and achieve a simple activation method, good infection effect, and streamlined operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: preparation kit
[0049] (1) Preparation of HB-Tmax2.0-M / Hplate24
[0050] a) Dilute the anti-CD3 and anti-CD28 antibodies to 8ug / ml with pH 7.4 PBS buffer, add 500ul diluted antibody solution to each well of a 24-well plate with no surface treatment, and coat at 4°C for 14h;
[0051] b) Discard the antibody coating solution, block the 24-well plate with 1% BSA in PBS buffer, and block at room temperature for 45 minutes;
[0052] c) Discard the blocking solution, dry it at 37°C for 3 hours;
[0053] d) Put it into an aluminum foil bag, add a desiccant, seal it and label it, and store it at 4°C.
[0054] (2) Assembly:
[0055] Assemble the recombinant retrovirus (HB-RV3) and HB-Tmax2.0-M / Hplate24 described in the present invention into a cassette. The virus needs to be stored at -80°C, and the remaining components are stored at 2- 8°C.
[0056] The HB-RV3 was obtained by using HB-RV mutations.
Embodiment 2
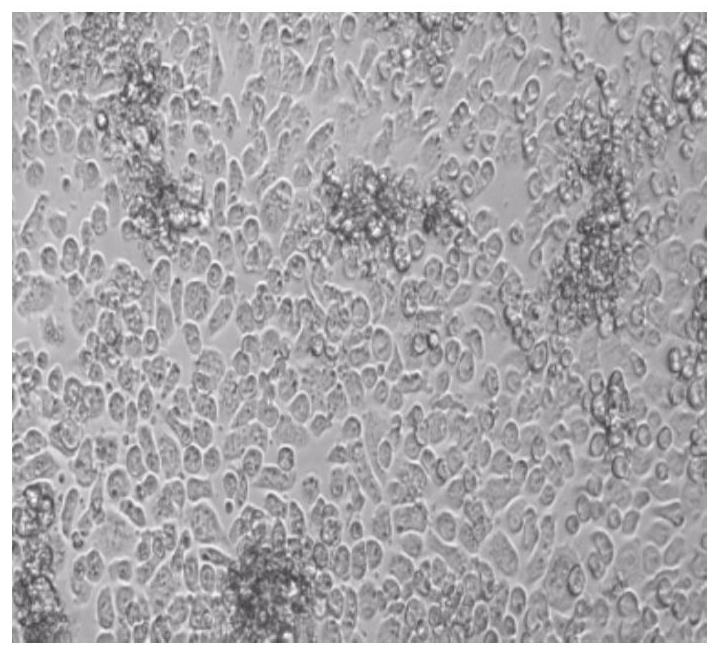
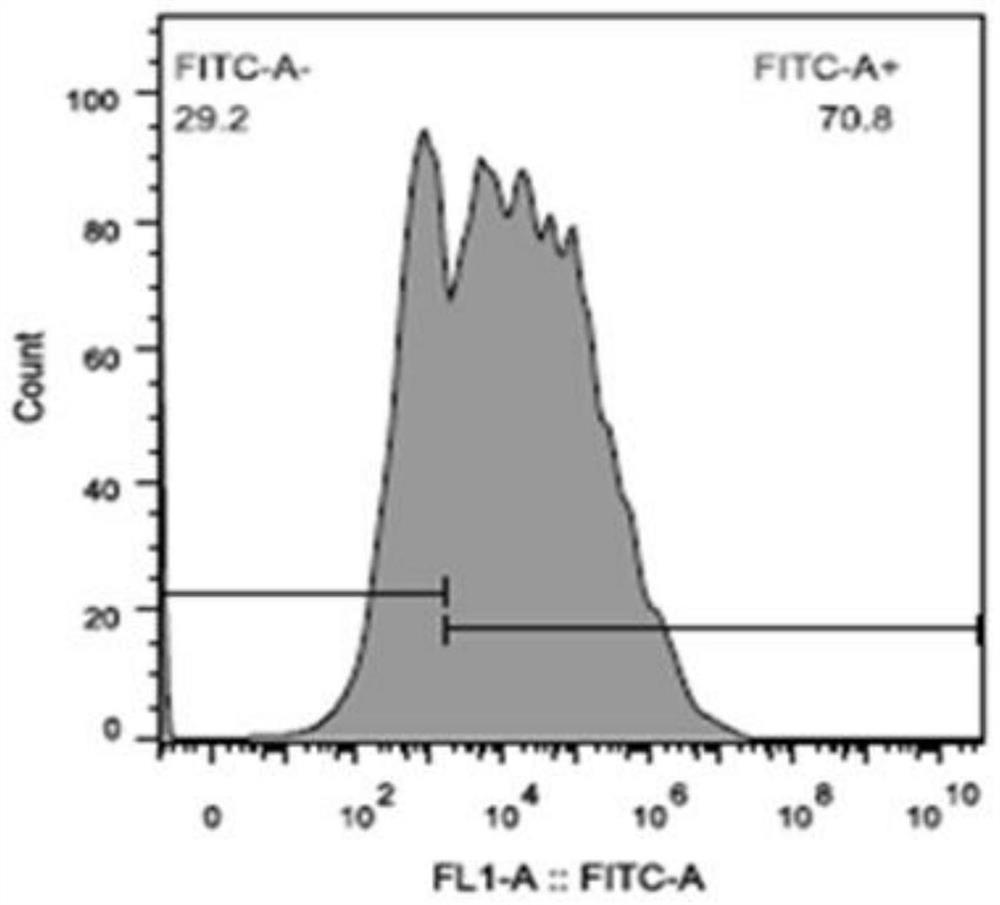
[0057] Example 2: Infection of mouse primary T cells
[0058] a) Isolation of mouse primary T cells ( Untouched TM Mouse CD4Cells Kit, 11416D, thermo), resuspended cells with complete medium containing 200U / ml rmIL-2 (R&D, 402-ML-020), 2*10 5 Every hole of each cell is paved with HB-Tmax2.0-Mplate24 (prepared in Example 1), the volume of the culture medium is maintained at 2ml, and 5% CO is put into 2 Incubator, activate at 37°C for 48h.
[0059] b) To observe the increase in the volume of activated cells, suck off 1.5ml of the medium and leave about 500ul of the medium and resuspend the cells by blowing, counting, and the number becomes 60% of the original, that is, 1.2*10 per well 5 Activated cells, add HB-RV3 virus, according to MOI25, that is, add 30ul of HB-RV3 virus per well, the virus titer is 1*10 8 TU / ml, add the virus and mix by pipetting.
[0060] c) Centrifuge the plate at 2000g for 2h at room temperature.
[0061] d) Return to 5% CO after centrifugation 2...
Embodiment 3
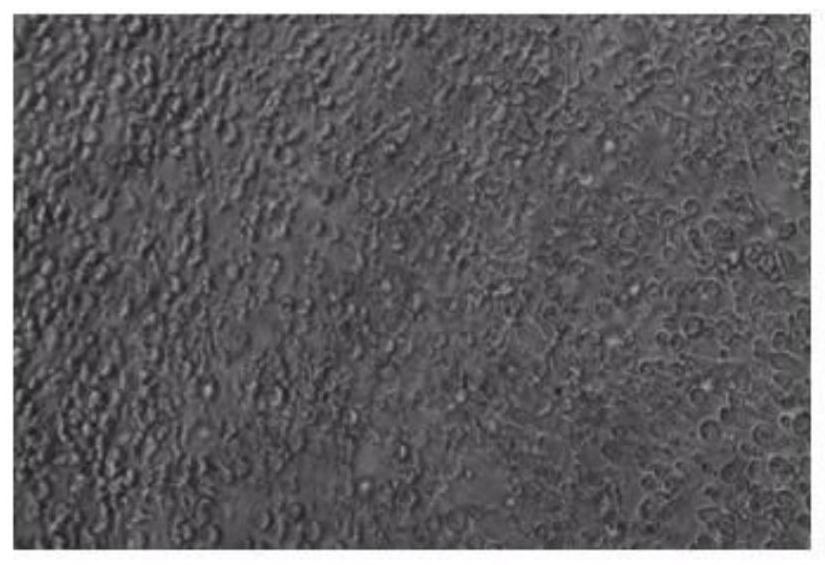
[0064] Example 3: Infection of human primary T cells
[0065] a) Isolation of human primary T cells ( Untouched TM Human CD4Cells Kit, 11352D, thermo), resuspended cells with complete medium containing 200U / ml rhIL-2 (R&D), 2*10 5 Spread HB-Tmax2.0-Hplate24 for each cell, keep the volume of the culture medium at 2ml, put in 5% CO 2 Incubator, activate at 37°C for 48h.
[0066] b) Observe the increase in the volume of activated cells, suck off 1.5ml of the medium, leave about 500ul of medium, and blow the cells to resuspend, count, and the number becomes 70% of the original, that is, 1.4*10 per well 5 For activated cells, add HB-RV3 virus, according to the MOI50, add the virus and mix well by pipetting.
[0067] c) Centrifuge the plate at 2000g for 2h at room temperature.
[0068] d) After centrifugation, put it back into the 5% CO2 incubator, infect at 37°C for 12 hours, discard 70% of the supernatant after 12 hours, add a complete medium containing 200U / ml rhIL-2 to ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com