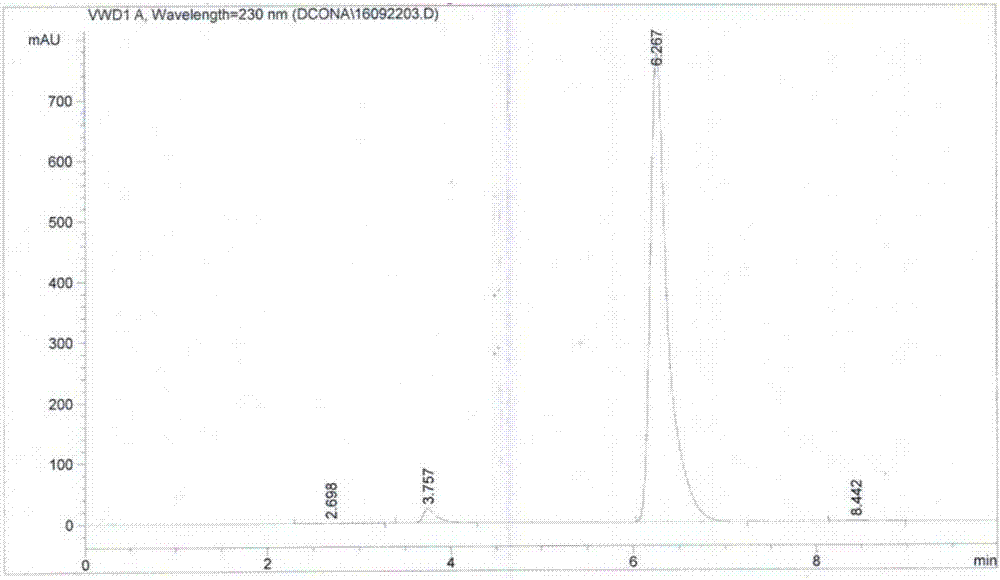
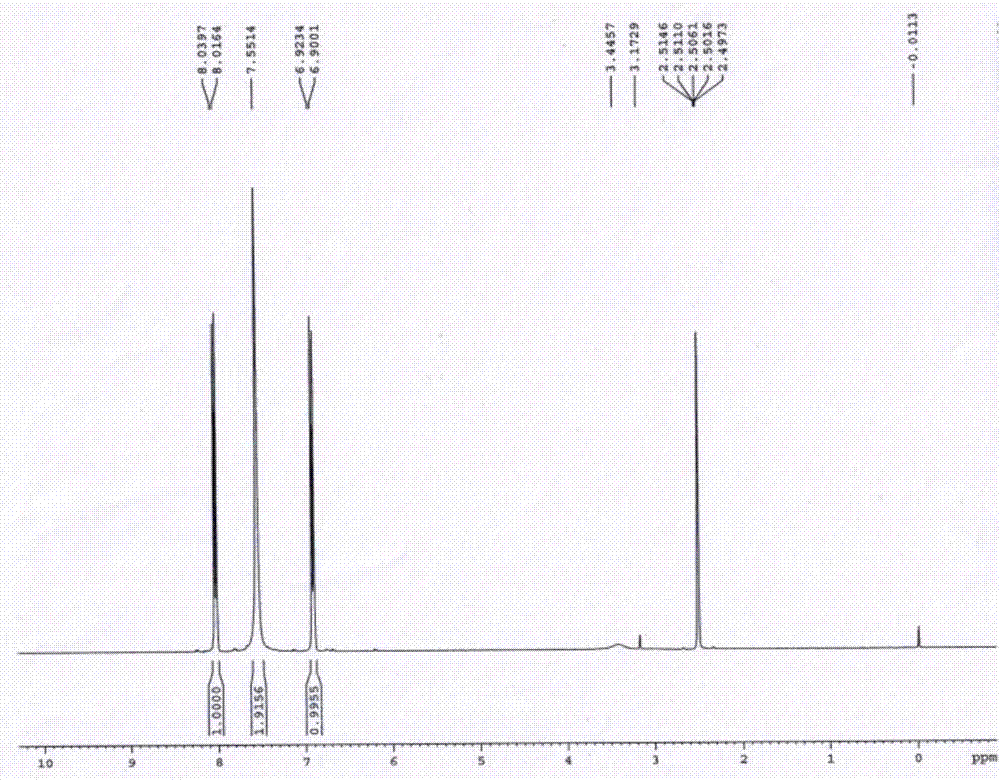
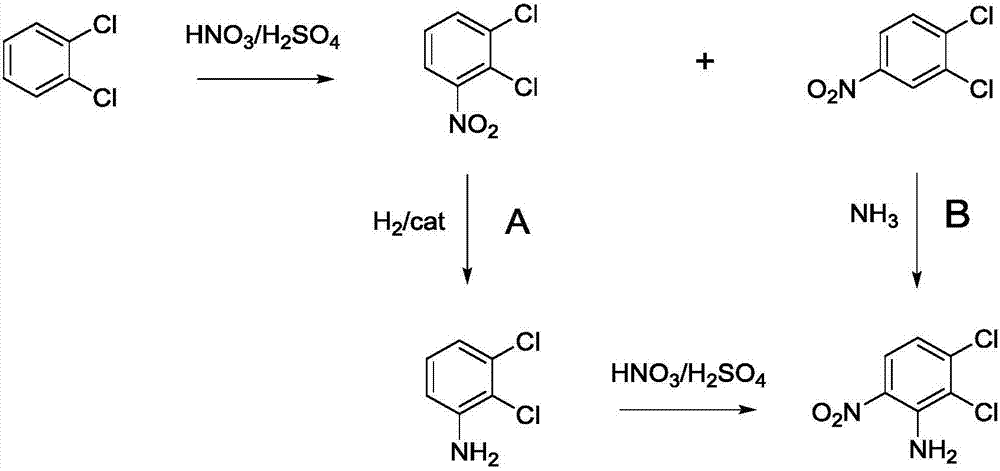
2, 3-dichloro-6-nitroaniline and preparation method thereof
A technology for nitroaniline and trichloronitrobenzene, which is applied in the preparation of nitro compounds, the preparation of amino compounds, chemical instruments and methods, etc., can solve the problems such as the inability to recover and apply sulfuric acid, the products are not easily separated, and the potential safety hazard is increased, and the invention is achieved. The effect of reducing environmental protection pressure, mild reaction conditions and reducing consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation method of 2,3-dichloro-6-nitroaniline comprises the following steps:
[0035] Preparation of S1, 2,3,4-trichloronitrobenzene: pump 200g of 98% sulfuric acid and 1816g of 1,2,3-trichlorobenzene into the nitration reactor, start stirring, and raise the temperature of the reactor to 45 ℃, after 1,2,3-trichlorobenzene is completely dissolved, pump 551g of 98% sulfuric acid and 696g of 95% nitric acid into the header tank, and the molar ratio of nitric acid to 1,2,3-trichlorobenzene is 1.05 : 1, sulfuric acid dehydration value D·V·S is 3.2. Add the mixed acid dropwise into the nitration reaction kettle for 20 minutes. After the dropwise addition, the reaction temperature is 50°C and the heat preservation reaction is 3 hours. The material layer was washed with water, and the water layer was separated to obtain 2239g of crude 2,3,4-trichloronitrobenzene, which was directly used for feeding in the next step;
[0036] S2, the preparation of 2,3-dichloro-6-nitro...
Embodiment 2
[0039] The preparation method of 2,3-dichloro-6-nitroaniline comprises the following steps:
[0040] S1. Preparation of 2,3,4-trichloronitrobenzene: pump 250g of 98% sulfuric acid and 2179g of 1,2,3-trichlorobenzene into the nitration reactor, start stirring, and raise the temperature of the reactor to 50°C After the 1,2,3-trichlorobenzene is completely dissolved, pump 749g of 98% sulfuric acid and 875g of 95% nitric acid into the header tank, and the molar ratio of nitric acid to 1,2,3-trichlorobenzene is 1.10: 1. Sulfuric acid dehydration value D·V·S is 3.5. Add the mixed acid dropwise into the nitration reaction kettle for 30 minutes. After the dropwise addition, the reaction temperature is 55°C and the heat preservation reaction is 2.5 hours. , the material layer was washed with water, and the water layer was separated to obtain 2688g 2,3,4-trichloronitrobenzene crude product, which was directly used for feeding in the next step;
[0041] Preparation of S2, 2,3-dichloro-...
Embodiment 3
[0044] The preparation method of 2,3-dichloro-6-nitroaniline comprises the following steps:
[0045] S1. Preparation of 2,3,4-trichloronitrobenzene: pump 400g of 98% sulfuric acid and 2724g of 1,2,3-trichlorobenzene into the nitration reactor, start stirring, and raise the temperature of the reactor to 55°C ,; After the 1,2,3-trichlorobenzene is completely dissolved, pump 1,009g of 98% sulfuric acid and 1,143g of 95% nitric acid into the header tank, and the molar ratio of nitric acid to 1,2,3-trichlorobenzene is 1.15 : 1, sulfuric acid dehydration value D·V·S is 3.9. Add the mixed acid dropwise into the nitration reaction kettle for 30 minutes. After the dropwise addition, the reaction temperature is 60°C and the heat preservation reaction is 2 hours. The material layer was washed with water, and the water layer was separated to obtain 3373g of crude 2,3,4-trichloronitrobenzene, which was directly used for feeding in the next step;
[0046] Preparation of S2, 2,3-dichloro-6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com