A kind of preparation method of 6-fluoro-1h-indole-4-carboxylic acid methyl ester
A technology of methyl formate and methyl nitrobenzoate, which is applied in the field of medicine, can solve the problems of low safety, high price, and difficult industrialization, and achieve the effects of high safety, low cost, and short reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
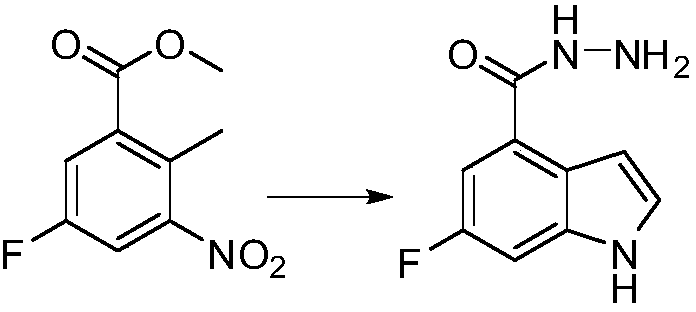
[0019] Example 1 Preparation of 6-fluoro-1H-indole-4-carboxylhydrazide
[0020]
[0021] In the reaction flask, add DMF (5L), 5-fluoro-2-methyl-3-nitrobenzoic acid methyl ester (1kg, 4.7mol), N,N-dimethylformamide dimethyl acetal ( 3kg, 25.2mol), triethylamine (250g, 2.47mol), after the addition, the temperature was raised to 100 degrees Celsius, the reaction was completed, the temperature was lowered, and rotary steaming was added, methanol (5 liters), 50% hydrazine hydrate (1.4kg, 14.1mol) were added , heated to 60 degrees Celsius, the reaction was completed, and filtered to obtain a white solid, 6-fluoro-1H-indole-4-carboxylhydrazide, 843g, yield: 93.1%.
Embodiment 2
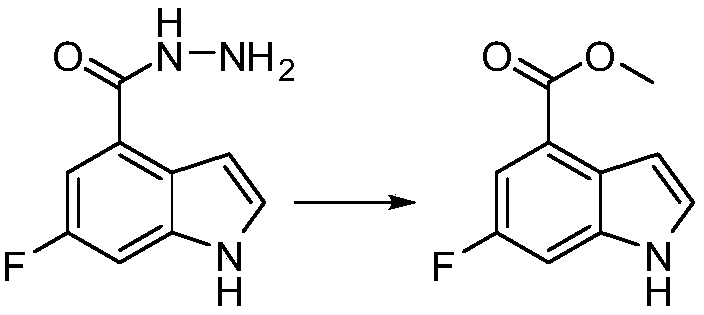
[0022] Example 2 Preparation of 6-fluoro-1H-indole-4-carboxylic acid methyl ester
[0023]
[0024] Add 6-fluoro-1H-indole-4-carboxylhydrazide (843g, 4.37mol) into the reaction flask, add methanol (1 liter), dichloromethane (5 liters), 2-iodobenzoic acid (1.47kg , 5.25mol), warming up to 40 degrees Celsius, stirring, the reaction is complete, adding 10% sodium sulfite aqueous solution, liquid separation, drying the organic layer, evaporating to dryness, recrystallizing with ethyl acetate and n-heptane to obtain the product 6-fluoro-1H- Indole-4-carboxylic acid methyl ester, 658g, yield: 78%.
[0025] 1H NMR (400MHz, 25°C, DMSO-d6):
[0026] 3.87(s,3H),6.9(dd,1H,J=0.8,3.1Hz),7.43-7.49(m,2H),7.51(d,1H,J=3.1Hz),11.47(s,br,1H) .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com