Terpyridine zinc benzoate complex and preparation condition thereof
A technology of zinc benzoate and terpyridine, which is applied in the direction of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems that there is no logical theory, long-wave zinc organic coordination polymer fluorescent materials are rare, etc., and achieve crystallization The effect of high purity, high thermal stability and simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
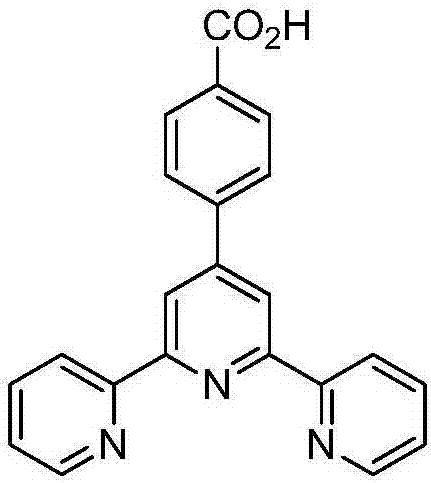
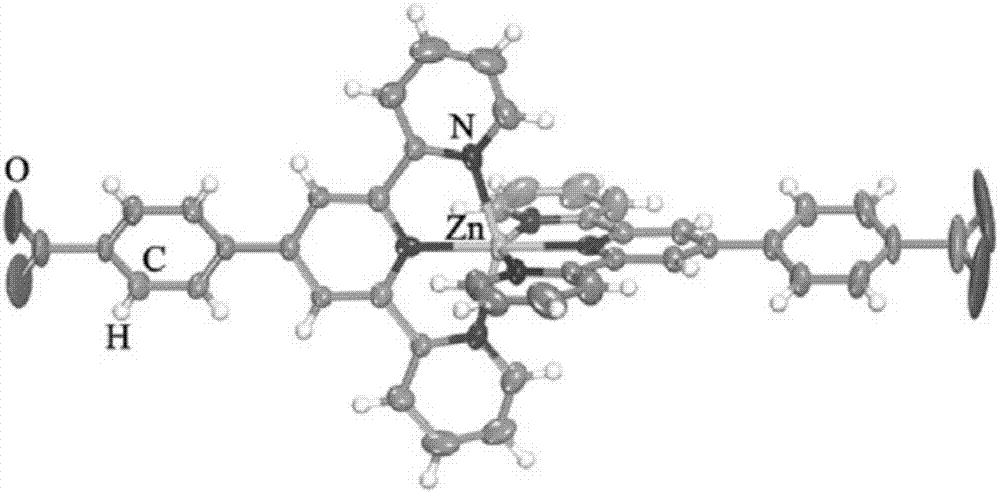
[0029] Example 1 Zinc complex of the present invention [Zn(tpb) 2 (Htpb) 2 ] preparation
[0030] a) Weigh the raw materials according to the following molar ratio, Htpb: Zn(NO 3 ) 2 :DMF:H 2 O: HNO 3 The molar ratio is 1:2:516:8888:18, and in a 50mL glass beaker, mix and stir for 0.5 hours;
[0031] b) Transfer the mixture obtained in step a) into a 25 mL reaction kettle, and react at 140° C. for 3 days;
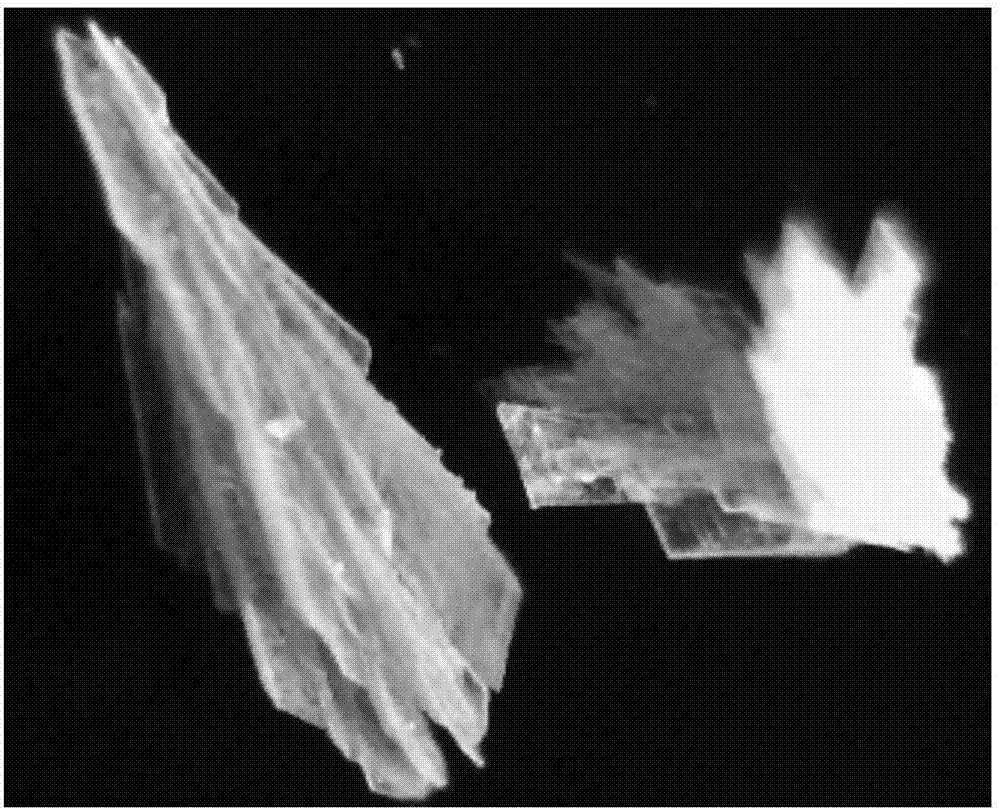
[0032] c) Naturally cooled to room temperature, yellow crystals were observed, filtered, washed with DMF and water in turn, and naturally dried to obtain the target product.
[0033] Use Perkin-Elmer2400 elemental analyzer to carry out elemental analysis on C, H, N elements of the target product, calculated value (%): C, 71.93; H, 4.02; N, 11.18; Actual measurement (%): C, 71.87; H, 4.03; N, 11.21.
[0034] Using a Nicolet Impact 410FTIR spectrometer with KBr as substrate at 400-4000cm -1 Infrared analysis of the target product within the range, FT-IR (KBr, cm -1 ...
Embodiment 2
[0039] Example 2 The zinc complex of the present invention [Zn(tpb) 2 (Htpb) 2 ] preparation
[0040] a) Weigh the raw materials according to the following molar ratio, Htpb: Zn(NO 3 ) 2 :DMF:H 2 O: HNO 3 The molar ratio is 1:1:516:8888:12, placed in a 50mL glass beaker, mixed and stirred for 1 hour;
[0041] b) Transfer the mixture obtained in step a) into a 25 mL reaction kettle, and react at 130° C. for 3 days;
[0042] c) Naturally cooled to room temperature, light yellow crystals were observed, filtered, washed with DMF and water in turn, and naturally dried to obtain the target product.
[0043] The target product was characterized by elemental analysis, infrared, fluorescence, and X-ray powder diffraction under the same conditions as in Example 1. The results were similar to those in Example 1. The target product obtained in Example 2 was the same as that obtained in Example 1. The sample is of high purity, the results are shown in Figure 5 .
[0044] Repeatin...
Embodiment 3
[0045] Example 3 The zinc complex of the present invention [Zn(tpb) 2 (Htpb) 2 ] preparation
[0046] a) Weigh the raw materials according to the following molar ratio, Htpb: Zn(NO 3 ) 2 :DMF:H 2 O: HNO 3 The molar ratio is 1:1:774:7778:15, placed in a 50mL glass beaker, mixed and stirred for 0.5h;
[0047] b) Transfer the mixture obtained in step a) into a 25 mL reaction kettle, and react at 140° C. for 2 days;
[0048] c) Naturally cooled to room temperature, light yellow crystals were observed, filtered, washed with DMF and water in turn, and naturally dried to obtain the target product.
[0049] The target product was characterized by elemental analysis, infrared, fluorescence, and X-ray powder diffraction under the same conditions as in Example 1. The results were similar to those in Example 1. The target product obtained in Example 3 was the same as that obtained in Example 1. The sample is of high purity, the results are shown in Figure 5 .
[0050] Repeating ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com