Novel self-paced and self-stirred reactor for chloride and concentrated sulfuric acid reaction for preparing hydrogen chloride and method
A concentrated sulfuric acid and chloride technology, applied in chemical/physical/physical-chemical fixed reactors, preparation with chlorides, chemical instruments and methods, etc. problems, to achieve the effect of reducing production costs, reducing emissions, and facilitating industrial implementation and operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
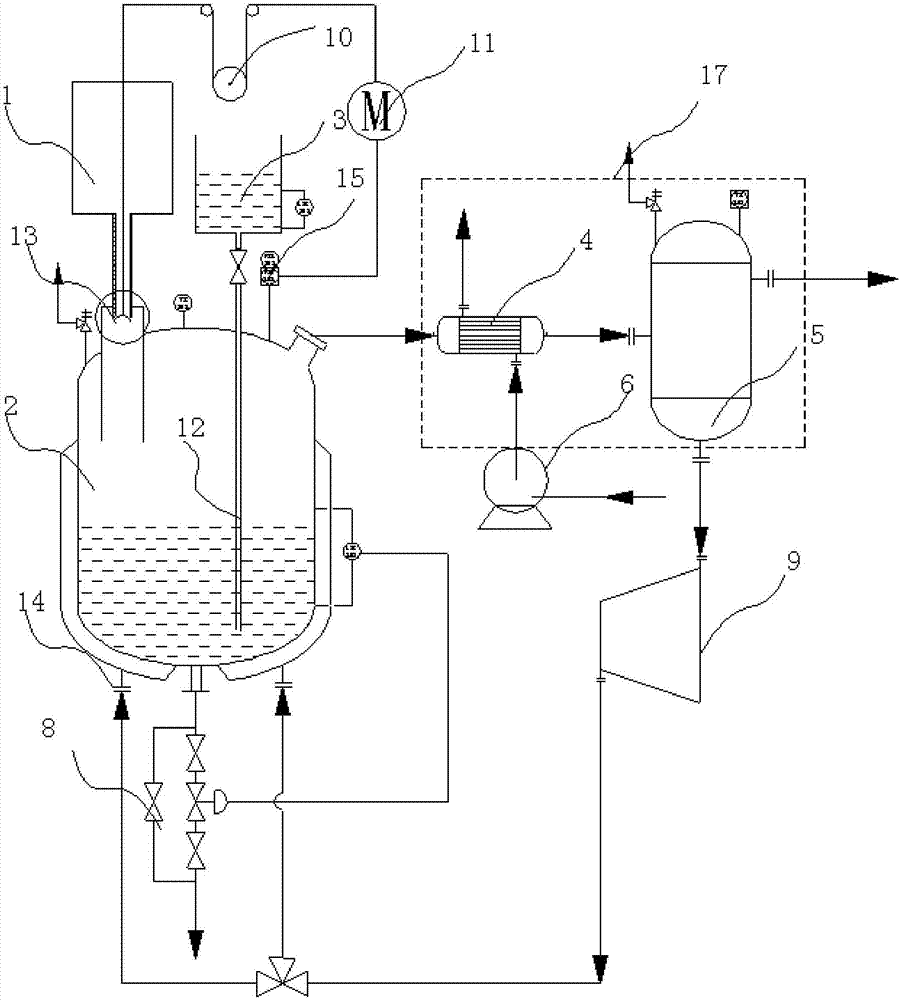
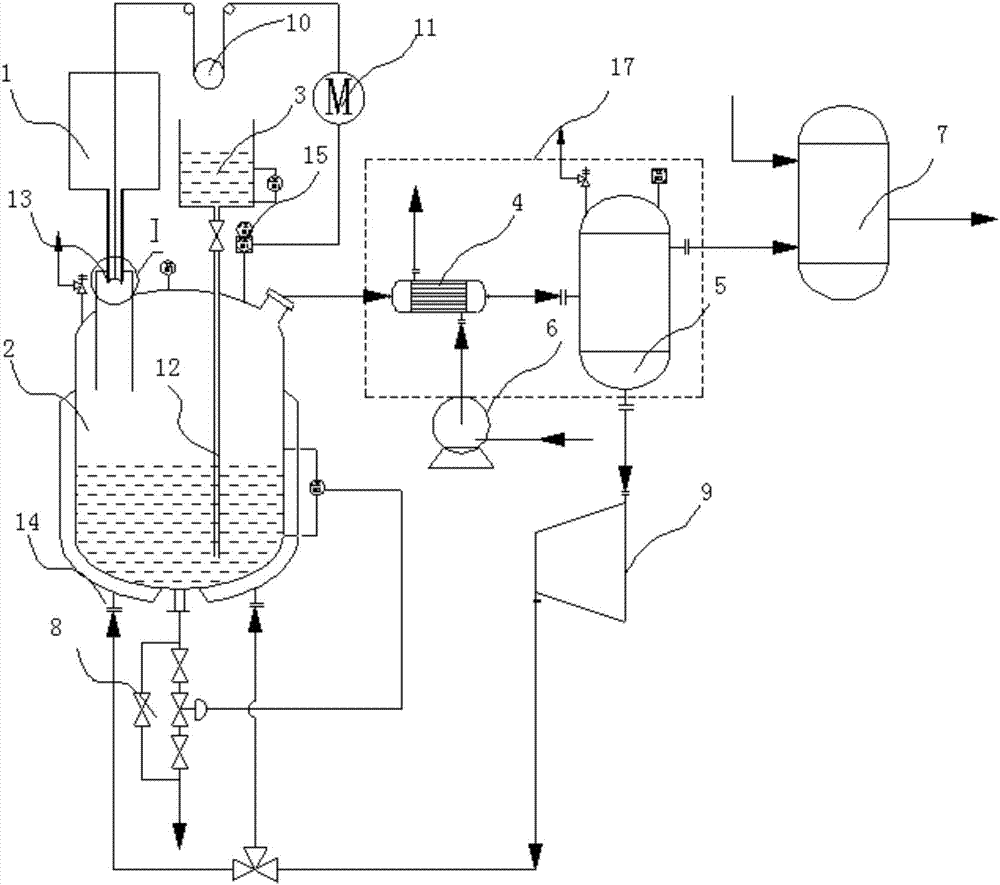
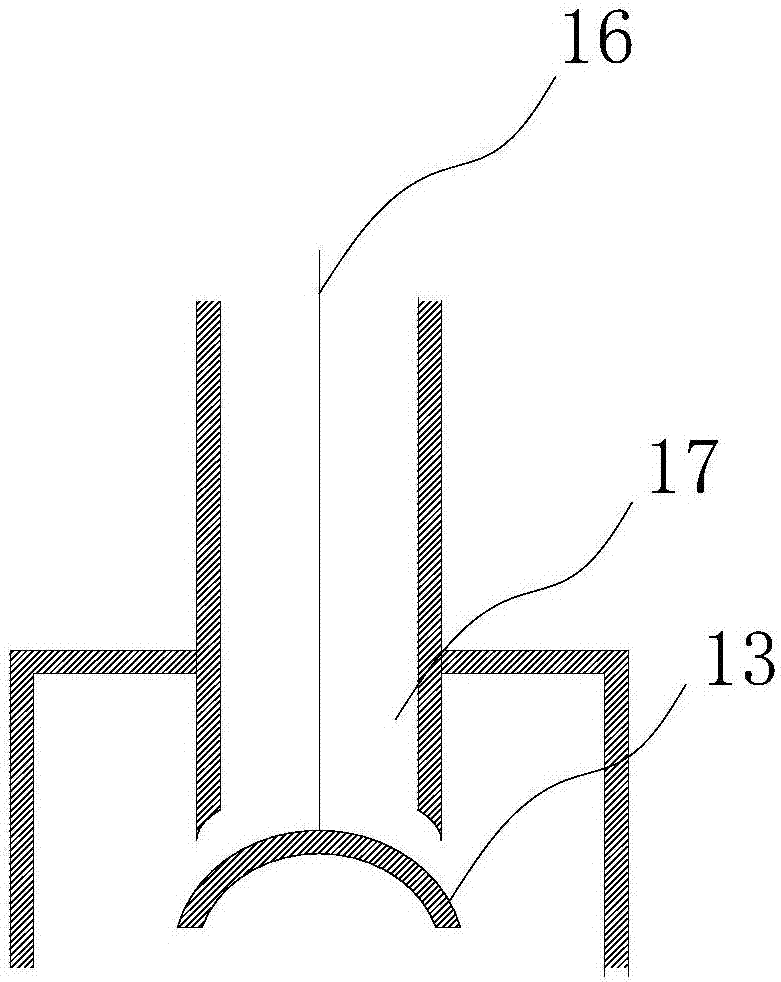
[0047] The reactor structure provided by the invention is as figure 1 As shown, there is a reactor 2, which is used to react solid chloride and concentrated sulfuric acid to generate hydrogen chloride, wherein the solid chloride can be sodium chloride, potassium chloride, ammonium chloride, etc.
[0048] The reaction kettle 2 is provided with a solid chloride raw material tank 1 for storing solid chloride and feeding it into the reaction kettle 2 .
[0049]A concentrated sulfuric acid storage tank 3 is also arranged on the reaction kettle 2 for storing the concentrated sulfuric acid and adding it dropwise into the reaction kettle 2 . In order to ensure a constant liquid level pressure in the storage tank, it is necessary to control the liquid level of the concentrated sulfuric acid to be constant. The concentrated sulfuric acid can be kept at a set liquid level through a conventional constant liquid level automatic control system. At present, the water level automatic control ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com