Fluorescent probe and application thereof to detection of explosives as well as preparation
A fluorescent probe and reaction technology, applied in the field of fluorescent probes, achieves the effects of convenient post-processing, low cost investment, and simple synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
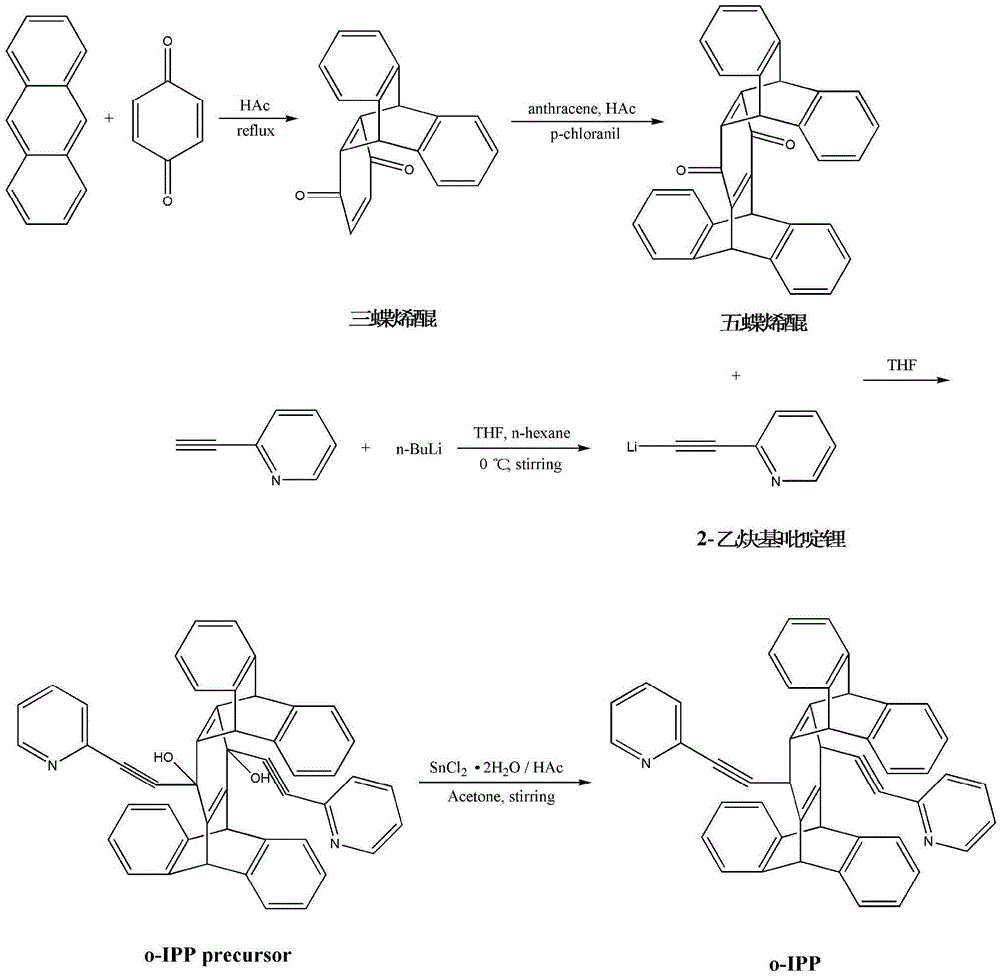
[0029] The synthesis of embodiment 1 triptylenoquinone:
[0030] Add 0.41g (2.30mmol) of anthracene, 1.39g (12.86mmol) of p-benzoquinone and 40mL of acetic acid into a 100mL three-necked flask. Start the stirrer, heat to reflux for 6 hours, after the reaction is cooled to room temperature, filter with suction, wash the filter residue with hot water, and dry to obtain 0.37g (1.30mmol) triptylenoquinone with a yield of 56.6%. by mass spectrometry, 1 H-NMR and 12 The C-NMR spectrum confirmed that the product was triptylenoquinone.
Embodiment 2
[0031] The synthesis of embodiment 2 pentaphenylenone:
[0032] Add 0.18g (1.00mmol) anthracene, 0.28g (1.00mmol) triptylenone, 0.25g (1.00mmol) chloranil and 60mL acetic acid into a 100mL three-necked flask. Start the stirrer, heat to reflux for 36 hours, cool the reaction to room temperature, filter with suction, wash the filter residue with ether, and dry to obtain 0.26g (0.57mmol) pentadecenequinone with a yield of 56.5%. by mass spectrometry, 1 H-NMR and 12 The C-NMR nuclear magnetic spectrum confirms that the product is pentastylenoquinone.
Embodiment 3
[0033] Synthesis of Example 3 2-ethynylpyridinium (compound IV):
[0034] A 10 mL three-neck flask was placed in an ice-water bath and flushed with nitrogen, and 0.5 mL (4.56 mmol) of 2-ethynylpyridine and 5 mL of anhydrous and oxygen-free tetrahydrofuran were added. Start the stirrer and cool the reactant to 0°C, and add 1.9 mL of 2.7M n-butyllithium / n-hexane solution dropwise under stirring and nitrogen protection to obtain a solution containing compound IV. It was confirmed by mass spectrometry that the product contained compound IV.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com