External compound tincture for treating dermatitis and preparation method of such external compound tincture
A technology for tinctures and compound prescriptions, which is applied in the field of compound external tinctures and their preparation, can solve the problems of exceeding the standard of related substances, affecting the safety and effectiveness of medication, and achieve the effects of enhancing stability and improving performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
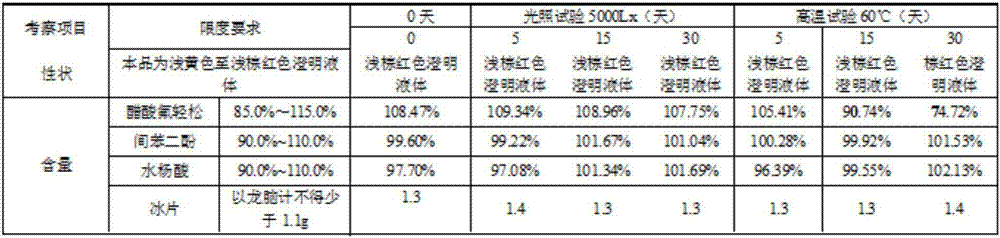
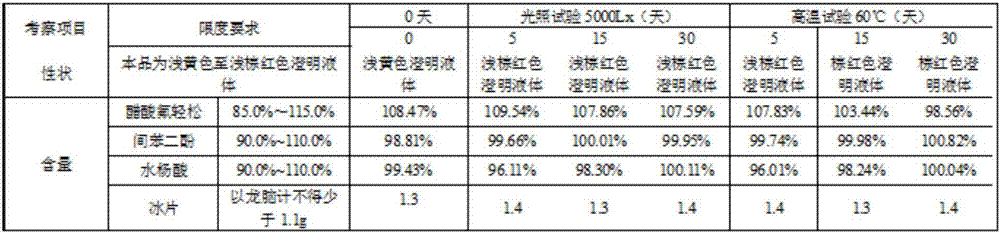
[0018] Embodiment 1: the compound external tincture for the treatment of dermatitis, its composition raw material comprises fluocinolone acetate, salicylic acid, resorcinol, borneol, clathrate, cosolvent, antioxidant, pH regulator and solvent; In parts by weight In total, each 1000g tincture contains the following active ingredients: fluocinolone acetate 0.1-10g, salicylic acid 5-100g, resorcinol 20-200g, borneol 5-50g, clathrate 1-10g, cosolvent 2-40g , 0.30-0.4g of antioxidant, 2-10g of pH regulator and 200-800g of solvent.
[0019] The inclusion compound is cyclodextrin, hydroquinone, urea, deoxycholic acid, methyl cyclodextrin, hydroxypropyl cyclodextrin, hydroxyethyl cyclodextrin, cyclodextrin polymer, ethyl One or more of cyclodextrin and linear cyclodextrin.
[0020] The co-solvents are organic acids and their salts such as benzoic acid, salicylic acid and its sodium salt, glycyrrhizic acid, etc., amides or amine compounds such as nicotinamide, acetamide, diethylamine,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com