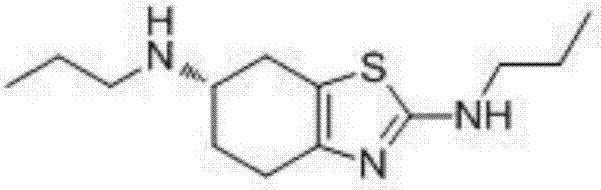
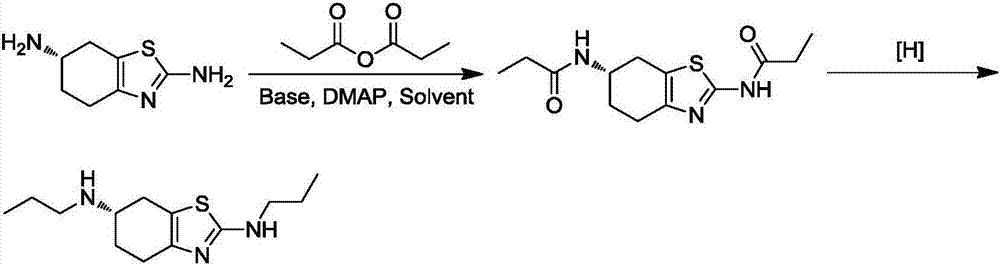
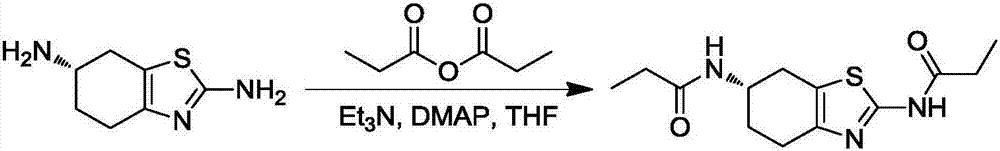
Synthetic method of pramipexole impurity B
A technology of pramipexole and synthesis method is applied in the field of preparation of pramipexole impurities to achieve the effects of simple process route and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Step 1: Synthesis of (S)-N,N'-(4,5,6,7-tetrahydrobenzothiazole-2,6-diyl)dipropionamide
[0023]
[0024] At room temperature, (S)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole (5.00g, 29.54mmol), triethylamine (8.97g, 88.63 mmol), propionic anhydride (9.61 g, 73.86 mmol) and DMAP (361 mg, 2.95 mmol). After the addition, the temperature of the system was raised to reflux for 2 hours. TLC detects that the reaction of raw materials is complete. Concentrate the reaction system to dryness, add 10 g of water and 40 g of ethyl acetate, stir vigorously for 3 hours, filter, and wash the filter cake with a little water and ethyl acetate. The obtained solid was transferred to an air blower at 60° C. and dried to constant weight to obtain 4.3 g with a yield of 51%.
[0025] Step 2: (S)-N 2 ,N 6 -Synthesis of dipropyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine
[0026]
[0027] Add (S)-N,N'-(4,5,6,7-tetrahydrobenzothiazole-2,6-diyl)dipropionamide (1.00g, 3.55mmol) to tetr...
Embodiment 2
[0029] Step 1: Synthesis of (S)-N,N'-(4,5,6,7-tetrahydrobenzothiazole-2,6-diyl)dipropionamide
[0030]
[0031] Add (S)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole (5.00g, 29.54mmol), pyridine to 1,4-dioxane (50mL) at room temperature (11.53g, 88.63mmol), propionic anhydride (9.61g, 73.86mmol) and DMAP (361mg, 2.95mmol). After the addition, the temperature of the system was raised to reflux for 2 hours. TLC detects that the reaction of raw materials is complete. Concentrate the reaction system to dryness, add 10 g of water and 40 g of ethyl acetate, stir vigorously for 3 hours, filter, and wash the filter cake with a little water and ethyl acetate. The obtained solid was transferred to an air blower at 60° C. and dried to constant weight to obtain 3.8 g with a yield of 45.7%.
[0032] Step 2: (S)-N 2 ,N 6 -Synthesis of dipropyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine
[0033]
[0034] Add (S)-N,N'-(4,5,6,7-tetrahydrobenzothiazole-2,6-diyl)dipropane to 1,4-dioxa...
Embodiment 3
[0036] Step 1: Synthesis of (S)-N,N'-(4,5,6,7-tetrahydrobenzothiazole-2,6-diyl)dipropionamide
[0037]
[0038] At room temperature, (S)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole (5.00g, 29.54mmol), N,N-diisopropyl Ethylamine (11.45 g, 88.63 mmol), propionic anhydride (9.61 g, 73.86 mmol) and DMAP (361 mg, 2.95 mmol). After the addition, the temperature of the system was raised to reflux for 2 hours. TLC detects that the reaction of raw materials is complete. Concentrate the reaction system to dryness, add 10 g of water and 35 g of ethyl acetate, stir vigorously for 3 hours, filter, and wash the filter cake with a little water and ethyl acetate. The obtained solid was transferred to an air blower at 60° C. and dried to constant weight to obtain 4.8 g with a yield of 57.7%.
[0039] Step 2: (S)-N 2 ,N 6 -Synthesis of dipropyl-4,5,6,7-tetrahydrobenzothiazole-2,6-diamine
[0040]
[0041] Add (S)-N,N'-(4,5,6,7-tetrahydrobenzothiazole-2,6-diyl)dipropionamide (1.00g, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com