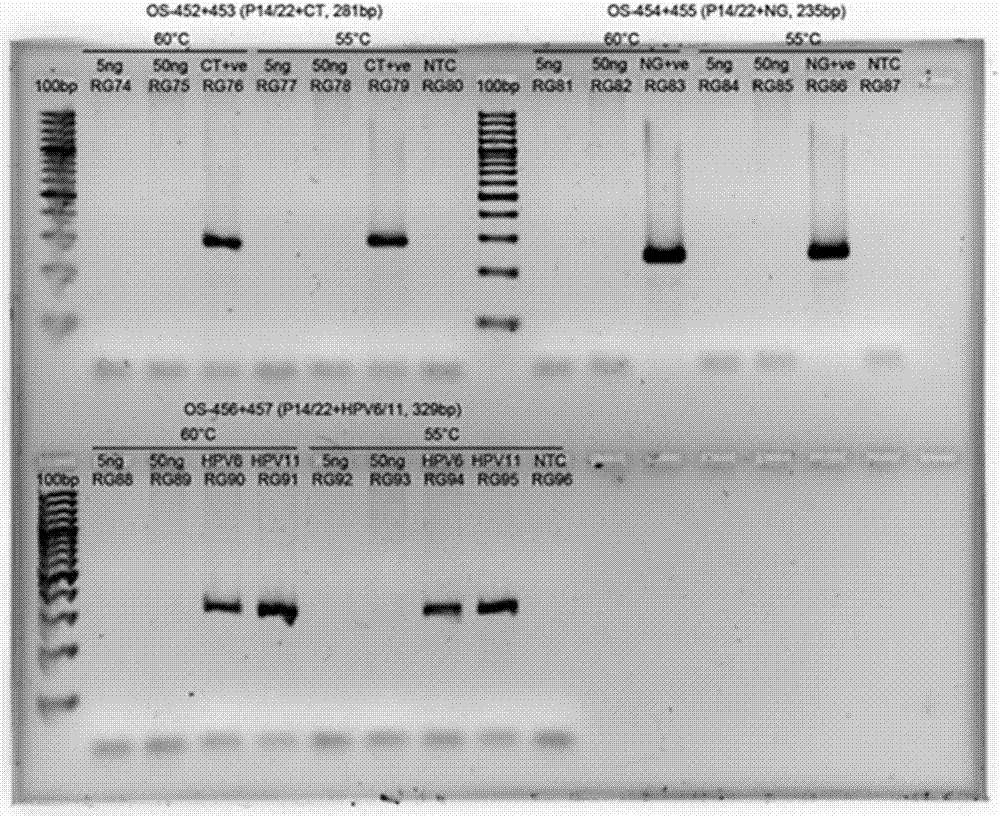
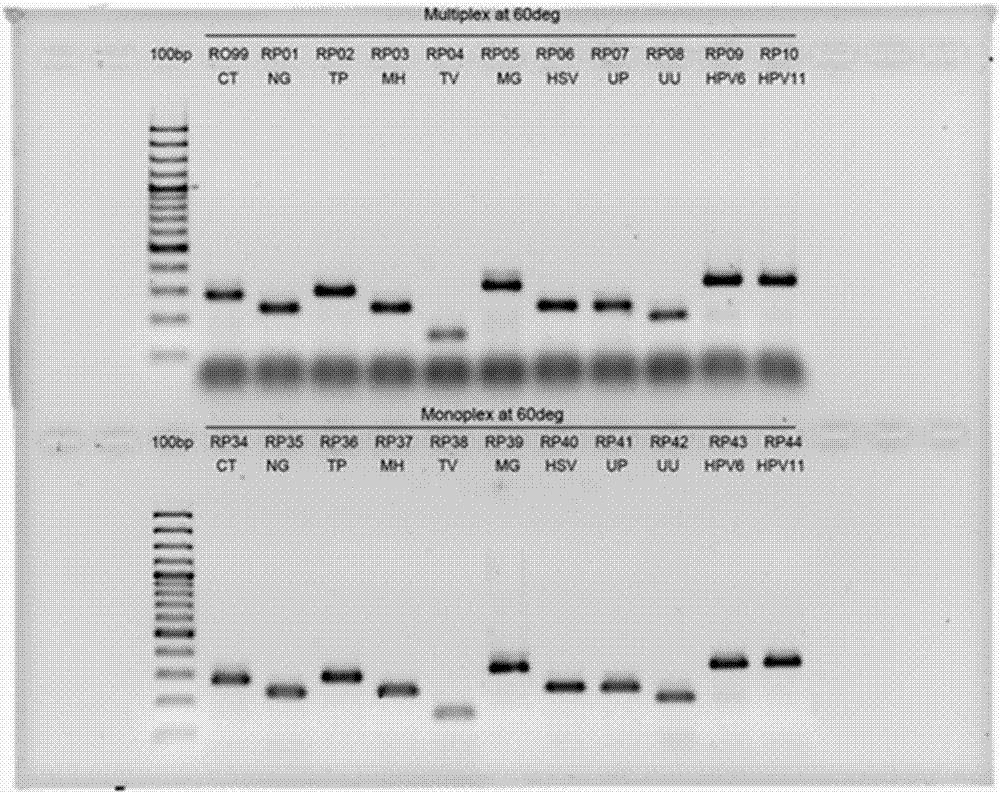
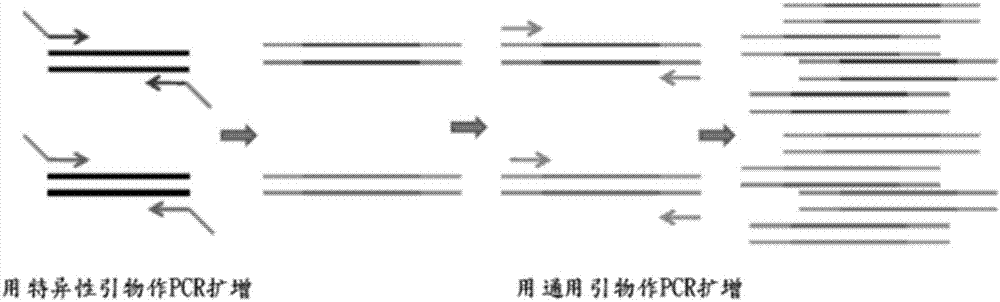
Rapid genotyping analysis and kits thereof
A technology of nucleic acid molecules and oligonucleotide probes, which is applied in the field of rapid and sensitive genotype identification and nucleic acid detection, and can solve problems such as limited membrane area and long incubation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] test program
[0076] Separation steps for DNA, PCR amplification, hybridization, detection of samples and result analysis, such as WO / 2011 / 139750 or related procedures and techniques known to those skilled in the art can be applied to the present invention. Various internal controls (IC) can be included in the test assay to verify DNA samples, PCR amplification and color development of results. The size and format of each baffle array can be tailored to individual needs, or to optimize chamber usage for maximum cost savings.
Embodiment 2
[0078] Genotyping MDR-TB
[0079] In one embodiment, the present invention provides methods and kits for genotyping DR-MTB. The method and the kit adopt polymerase chain reaction (PCR) and "flow-through" hybridization technology for detecting Mycobacterium tuberculosis (MTB) resistant to rifampicin (RIF) and isoniazid (INH) . The corresponding primers used to amplify the rpoB gene, katG gene and INHA gene have been confirmed not to cross-react with the human genome. RS-IAC is an internal control primer that does not target the human or MTB genome and is used to monitor the PCR amplification process. The probes used to detect drug-resistant gene mutations included the rpoB gene (D516V, D516G, H526D, H526Y, H526L1, S531L, and S531W), the katG gene (S315T1 and S315T2), and the inhA gene (-15TC / T). There are also five probes for the detection of mutations in wild-type MTB, which help to verify whether the PCR amplification reactions of the rpoB gene, katG gene, and inhA gene ...
Embodiment 3
[0094] Beta Med Genotyping
[0095] The present invention provides a systematic detection of β-globulin gene mutation, said gene mutation includes TATA-28 (A>G), TATA-29 (A>G), initiation codon (G>A), Detection of codon 5 (-CT), codon 8 / 9 (+G), codon 15 (G>A), codon 16 (-C), codon 17 (A>T), codon 19 ( A>G), codon 26 (G>A) (hemoglobin E), codon 27 / 28 (+three), codon 30G>C, IVS1.1 (G>T), IVS1.1 (G> A), IVS1.5(G>C), codon 41 / 42(-TCTT), codon 43(G>T), codon 71 / 72(+A), IVS2.1(G>A), IVS2.654(C>T) and 619bp deletion.
[0096] The present invention also provides a method for detecting β-globulin mutation in a subject sample, the method comprising the following steps: (a) obtaining a sample comprising a template nucleic acid molecule from the subject; A group consisting of primers of 201-205 amplifies the template nucleic acid molecule, thereby generating an amplification product; and (c) the amplification product is composed of an oligonucleotide probe selected from the sequence ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com