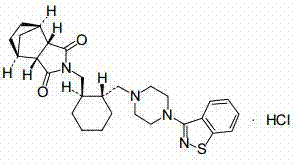
Preparation method for lurasidone hydrochloride
A technology of lurasidone hydrochloride and salt formation, which is applied in the field of drug synthesis, can solve the problems of unsuitability for large-scale industrial production, high production process cost, and low yield, and achieve low toxicity, low production process cost, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
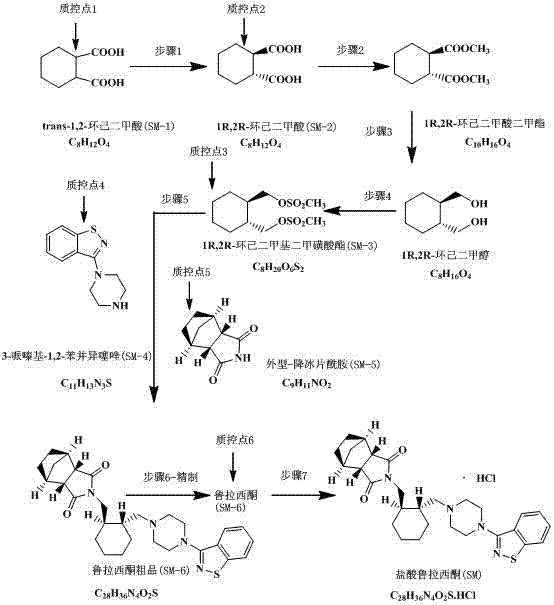
[0024] (1) Using trans-1,2-cyclohexanedicarboxylic acid (SM-1) as raw material to obtain 1R,2R-cyclohexanedicarboxylic acid (SM-2); trans-1,2-cyclohexanedicarboxylic acid (SM-2); Alkanedioic acid and R-(+)-α-phenethylamine reacted at 5°C to form a salt, filtered and dried at room temperature; then recrystallized in a hot ethanol / toluene (1:1) mixed solvent 1 Finally, add hydrochloric acid to free, and extract with ether to obtain (R, R)-1,2-cyclohexanedicarboxylic acid.
[0025] (2) Dimethyl 1R, 2R-cyclohexanedicarboxylate was obtained by methyl esterification; 1R, 2R-cyclohexanedicarboxylic acid was added to 400ml of methanol, concentrated sulfuric acid was added dropwise at room temperature and then heated to reflux for 5 hours of reaction.
[0026] (3) Reduction to obtain 1R, 2R-cyclohexanedimethanol; add 1R, 2R-dimethyl cycloadipate, THF, and NaBH4 into the reaction flask, heat to 45°C, slowly add methanol dropwise, and reflux after dropping After 2.5h, 1R, 2R-cyclohexane...
Embodiment 2
[0032] (1) Using trans-1,2-cyclohexanedicarboxylic acid (SM-1) as raw material to obtain 1R,2R-cyclohexanedicarboxylic acid (SM-2); trans-1,2-cyclohexanedicarboxylic acid (SM-2); Alkanedioic acid and R-(+)-α-phenethylamine reacted at 10°C to form a salt, filtered and dried at room temperature; then recrystallized in a hot ethanol / toluene (1:1) mixed solvent 2 Finally, add hydrochloric acid to free, and extract with ether to obtain (R, R)-1,2-cyclohexanedicarboxylic acid.
[0033] (2) Methyl esterification to obtain dimethyl 1R, 2R-cyclohexanedicarboxylate; add 1R, 2R-cyclohexanedicarboxylic acid to 500ml of methanol, add concentrated sulfuric acid dropwise at room temperature and heat to reflux for 6 hours of reaction.
[0034] (3) Reduction to obtain 1R, 2R-cyclohexanedimethanol; add 1R, 2R-dimethyl cycloadipate, THF, and NaBH4 into the reaction bottle, heat to 40°C, slowly add methanol dropwise, and reflux after dropping 3h, 1R, 2R-cyclohexanedimethanol was obtained after p...
Embodiment 3
[0040] (1) Using trans-1,2-cyclohexanedicarboxylic acid (SM-1) as raw material to obtain 1R,2R-cyclohexanedicarboxylic acid (SM-2); trans-1,2-cyclohexanedicarboxylic acid (SM-2); Alkanedioic acid and R-(+)-α-phenethylamine reacted at 25°C to form a salt, filtered and dried at room temperature; then recrystallized in hot ethanol / toluene (1:1) mixed solvent3 Finally, add hydrochloric acid to free, and extract with ether to obtain (R, R)-1,2-cyclohexanedicarboxylic acid.
[0041] (2) Methyl esterification to obtain dimethyl 1R, 2R-cyclohexanedicarboxylate; add 1R, 2R-cyclohexanedicarboxylic acid to 300ml of methanol, add concentrated sulfuric acid dropwise at room temperature and heat to reflux for 4 hours of reaction.
[0042] (3) Reduction to obtain 1R, 2R-cyclohexanedimethanol; add 1R, 2R-dimethyl cycloadipate, THF, and NaBH4 into the reaction flask, heat to 50°C, slowly add methanol dropwise, and reflux after dropping 2h, 1R, 2R-cyclohexanedimethanol was obtained after post-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com