Application of hesperetin to preparation of medicines for preventing and treating diabetes
A technology of hesperetin and diabetes, which is applied in the new application field of hesperetin or hesperidin, and can solve the problems such as exacerbation of type II diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1: Inhibitory effect of hesperetin on α-glucosidase
[0068] The following experiments were carried out under the conditions of the substrate system containing hesperetin and without hesperetin.
[0069] 1. The substrate system contains hesperetin
[0070] 1. Experimental method
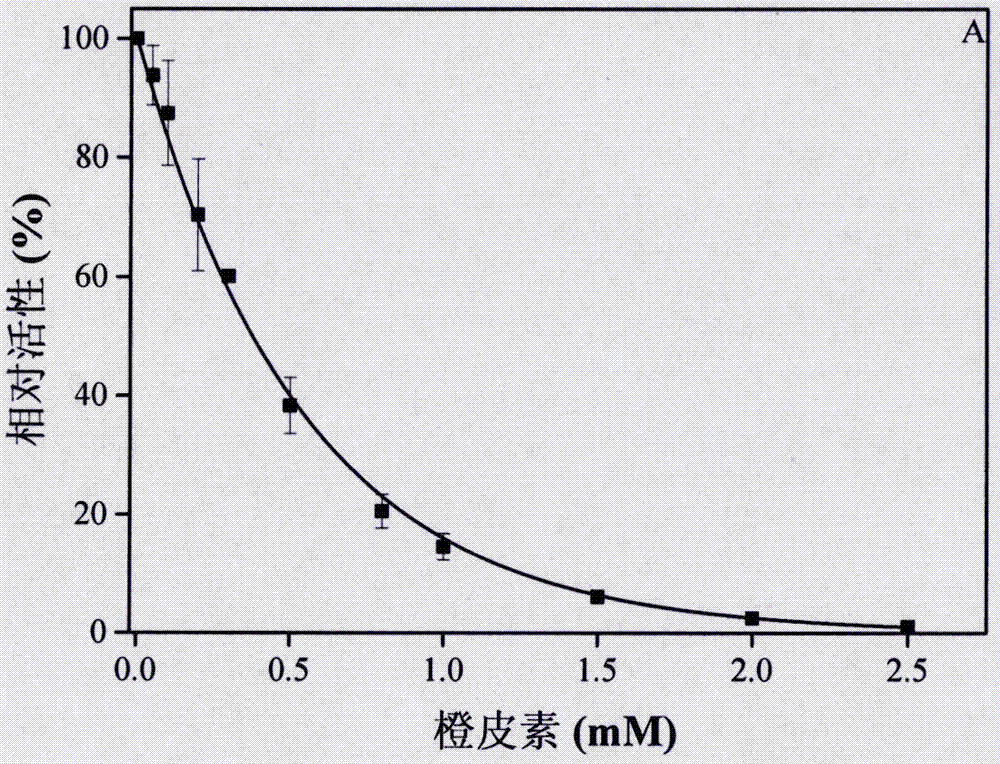
[0071] (1) The α-glucosidase solution is mixed with different concentrations of hesperetin solutions at a volume ratio of 20:1, and the concentration of hesperetin after mixing is 0mM, 0.1mM, 0.2mM, 0.3mM, 0.4mM, 0.5mM, 0.8mM, 1.0mM, 1.5mM, 2.0mM, 2.5mM, in a water bath at 25°C for 10min, and 11 parts of the mixture were obtained.
[0072] (2) 10 μL of each part of the above mixture was added to 1 ml of the substrate system, wherein the substrate system was prepared from pNPG solution and corresponding concentration of hesperetin solution at a volume ratio of 95:5. The purpose of adding hesperetin here is to ensure that after adding to the substrate system, the concentration of he...
Embodiment 2
[0087] Example 2: Verification experiment of inhibition reversibility
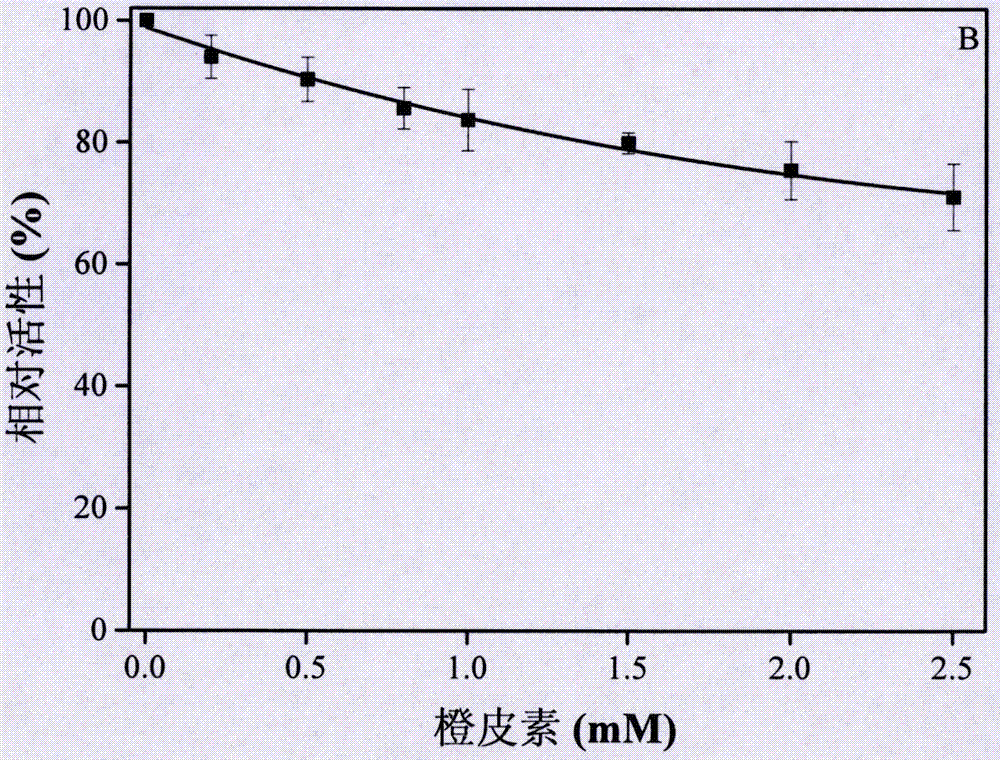
[0088] In order to detect and verify the reversibility of hesperetin as an α-glucosidase inhibitor, to further prove the experimental results of Example 1, the relationship between the enzyme residual activity and the enzyme concentration was made under the condition of changing the concentration of hesperetin.
[0089] 1. Experimental method
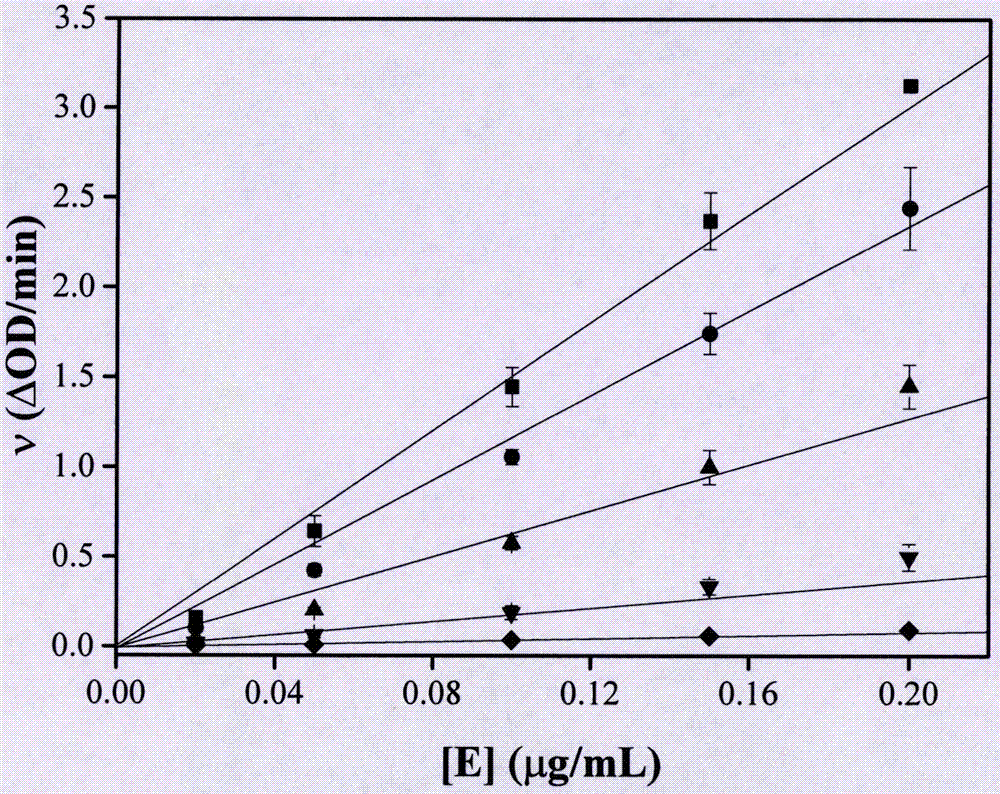
[0090] (1) The α-glucosidase solution was mixed with different concentrations of hesperetin solutions (hesperetin concentrations: 0mM, 0.2mM, 0.5mM, 1.0mM and 2.0mM) at a volume ratio of 20:1, at 25°C In a water bath for 10 min, 5 parts of the mixture were obtained.
[0091] (2) 10 μL of the above mixture was added to 1 ml of the substrate system, wherein the substrate system was prepared from pNPG solution and hesperetin solution of corresponding concentration at a volume ratio of 95:5. The initial reaction concentration of the substrate pNPG is 2.0 mM, and [E]...
Embodiment 3
[0098] Example 3: Time course kinetics of the inhibitory effect of hesperetin on α-glucosidase
[0099] 1. Experimental method
[0100] (1) The α-glucosidase solution is mixed with different concentrations of hesperetin solutions (0mM, 1mM, 4mM, 6mM, 10mM, 16mM) at a volume ratio of 20:1 to obtain 6 parts of the mixture, which are placed in a water bath at 25°C , specified time intervals: 1, 2, 3, 4, 5, 10, 15, 20, 25, 30min, respectively take 10μL of the above mixture and add it to the substrate system (1ml) corresponding to the concentration of hesperetin, The substrate system is obtained by preparing pNPG solution and hesperetin solution of corresponding concentration at a volume ratio of 95:5. The initial reaction concentration of the substrate pNPG was 2.0 mM, and that of α-glucosidase was 0.1 μM.
[0101] (2) Measure the activity change of α-glucosidase with an ultraviolet spectrophotometer under the condition of emission wavelength 400nm / min, and the measurement tem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com