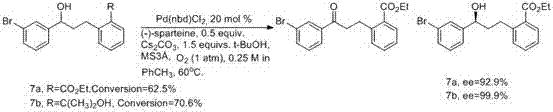
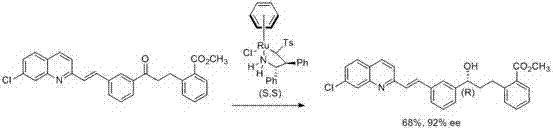
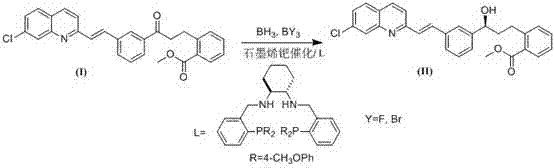
Method for synthesizing montrlukast sodium chiral alcohol intermediate by means of catalysis of graphene palladium copper
The technology of montelukast sodium and alkene palladium copper is applied in the field of synthesis of montelukast sodium intermediates, which can solve the problem of low ee value of product alcohol, great influence of chiral impurity reaction, and great difficulty in synthesizing chiral ligands of catalysts. and other problems, to achieve the effect of good chiral selectivity, simple operation, easy separation, recovery and reuse.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0018] Specific Example 1: [S-(E)]-2-[3-[3-[2-(7-chloro-2-quinolyl)vinyl]phenyl]-3-hydroxypropyl]benzoic acid Synthesis of methyl esters:
[0019] Take a dry 50 ml three-neck round bottom flask and add 590.1mg methyl (E)-2-(3-(3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl)-3-oxopropyl)benzoate, 20mg Graphene loaded palladium-copper nanocapsules containing palladium 10% mass fraction, add 10 milliliters of anhydrous N under the protection of nitrogen, N-dimethylformamide, keep the N of the reaction system 2 Protection, under ice-water bath cooling, control the temperature of the reaction solution at -2~0 o C, Add ligand 11.4mg(1S,2S)-N to the reaction solution 1 ,N 2 -bis(1-(2-(di-4-methoxyphenylphosphanyl) phenyl) ethyl) cyclohexane-1,2-diamine, stirred for 20 minutes, then added 268mg α-pinene (alpha-pinene), and added 0.18mL BBr drop by syringe 3 , then dropwise add 0.22mL borane tetrahydrofuran solution, and raise the temperature to 35 o C, react for 3.5 hours until the com...
specific Embodiment 2
[0020] Specific Example 2: [S-(E)]-2-[3-[3-[2-(7-chloro-2-quinolyl)vinyl]phenyl]-3-hydroxypropyl]benzoic acid Synthesis of methyl esters:
[0021] Take a dry 50 ml three-necked round bottom flask and add 590.1 mg methyl (E)-2-(3-(3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl)-3-oxopropyl)benzoate, 20mg of graphene-loaded palladium-copper nanocapsules containing 10% palladium by mass fraction, add 10 milliliters of anhydrous N under nitrogen protection, N-dimethylformamide, keep the N of the reaction system 2 Protection, under ice-water bath cooling, control the temperature of the reaction solution at -2~0 o C, add ligand 12.4mg (1S,2S)-N to the reaction solution 1 ,N 2 -bis(1-(2-(di-4-methoxyphenylphosphanyl) phenyl) ethyl)cyclohexane-1,2-diamine, stirred for 20 minutes, then added 278.2mg of α-pinene (alpha-pinene), dripped by syringe 0.26mL BBr 3 , then dropwise add 0.32mL borane tetrahydrofuran solution, and raise the temperature to 35 o C, react for 3.5 hours until the co...
specific Embodiment 3
[0022] Specific example 3, [S-(E)]-2-[3-[3-[2-(7-chloro-2-quinolyl) vinyl] phenyl]-3-hydroxypropyl] benzoic acid Synthesis of methyl esters:
[0023]Take a dry 100 ml three-neck round bottom flask and add 974.2 mg methyl (E)-2-(3-(3-(2-(7-chloroquinolin-2-yl)vinyl)phenyl)-3-oxopropyl)benzoate, 30mg of graphene-loaded palladium-copper nanocapsules containing palladium 10% mass fraction, add 30 milliliters of anhydrous N under the protection of nitrogen, N-dimethylformamide, keep the N of the reaction system 2 Protection, under ice-water bath cooling, control the temperature of the reaction solution at -2~0 o C, add ligand 19.2mg (1S,2S)-N to the reaction solution 1 ,N 2 -bis(1-(2-(di-4-methoxyphenylphosphanyl) phenyl) ethyl)cyclohexane-1,2-diamine, stirred for 20 minutes, then added 428.5mg α-pinene (alpha-pinene), dripped by syringe 0.31mL BBr 3 , then dropwise add 0.35mL borane tetrahydrofuran solution, and raise the temperature to 35 o C, react for 3.8 hours until the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com