Ramosetron hydrochloride effervescent tablet and preparation method thereof
A technology of ramosetron tablets and ramosetron bubbles, which is applied in the field of medicine and can solve the problems of instability, decreased stability and high bioavailability of the active ingredient ramosetron hydrochloride
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
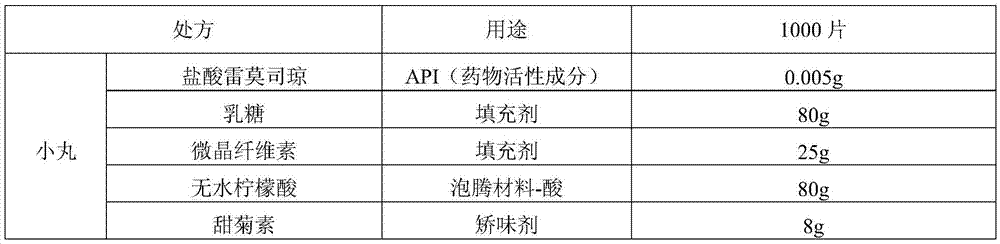
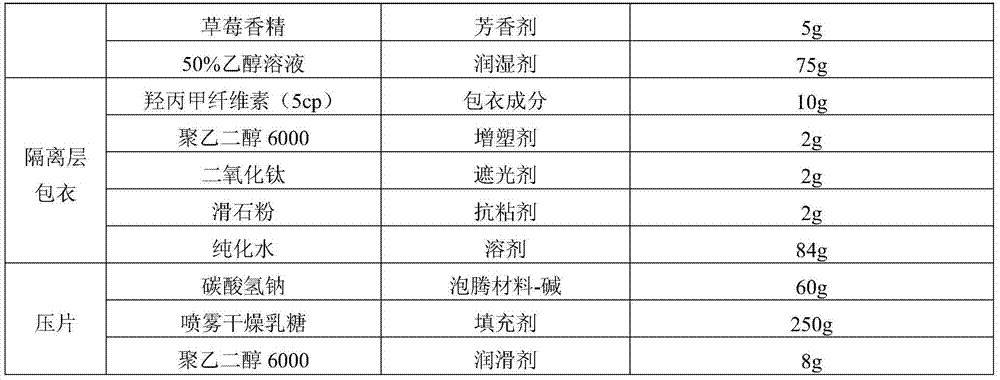
[0036] Ramosetron hydrochloride effervescent tablet of the present invention, prescription is as table 1.
[0037] Table 1
[0038]
[0039]
[0040] The preparation method of ramosetron hydrochloride effervescent tablet comprises steps:
[0041] (1) Preparation of pellets:
[0042] a. Dissolve ramosetron hydrochloride in 50w.t.% ethanol solution, add stevioside and strawberry essence in formula quantity, and then carry out wet granulation to the mixture of lactose, microcrystalline cellulose and anhydrous citric acid;
[0043] b. Pass the above-mentioned soft material through an extruder equipped with a 0.5mm sieve, and use a ball forming device to cut and round the extruded strip-shaped material on the friction plate into pellets, and dry at 50°C.
[0044] c. Prepare the coating solution for the isolation layer: prepare an appropriate amount of 10w.t.% hypromellose aqueous solution, then add polyethylene glycol 6000, titanium dioxide, and talcum powder, and stir even...
Embodiment 2
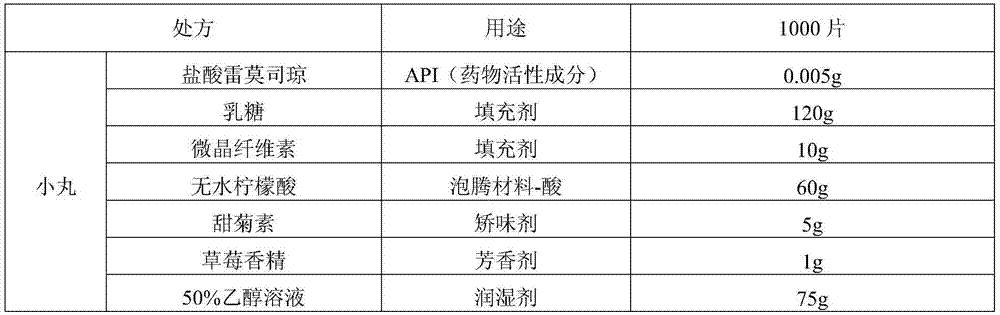
[0048] Ramosetron hydrochloride effervescent tablet of the present invention, prescription is as table 2.
[0049] Table 2
[0050]
[0051]
[0052] The preparation method of ramosetron hydrochloride effervescent tablet comprises steps:
[0053] (1) Preparation of pellets:
[0054]a. Dissolve ramosetron hydrochloride in 50w.t.% ethanol solution, add stevioside and strawberry essence in formula quantity, and then carry out wet granulation to the mixture of lactose, microcrystalline cellulose and anhydrous citric acid;
[0055] b. Pass the above-mentioned soft material through an extruder equipped with a 0.5mm sieve, and use a ball forming device to cut and round the extruded strip-shaped material on the friction plate into pellets, and dry at 50°C.
[0056] c. Prepare the coating solution for the isolation layer: prepare an appropriate amount of 7.5w.t.% hypromellose aqueous solution, then add polyethylene glycol 6000, titanium dioxide, and talcum powder, and stir even...
Embodiment 3
[0060] Ramosetron hydrochloride effervescent tablet of the present invention, prescription is as table 3.
[0061] table 3
[0062]
[0063]
[0064] The preparation method of ramosetron hydrochloride effervescent tablet comprises steps:
[0065] (1) Preparation of pellets:
[0066] a. Dissolve ramosetron hydrochloride in 50w.t.% ethanol solution, add stevioside and strawberry essence in formula quantity, and then wet the mixture of lactose, microcrystalline cellulose, anhydrous citric acid and tartaric acid grain;
[0067] b. Pass the above-mentioned soft material through an extruder equipped with a 0.5mm sieve, and use a ball forming device to cut and round the extruded strip-shaped material on the friction plate into pellets, and dry at 50°C.
[0068] c. Prepare the coating solution for the isolation layer: prepare an appropriate amount of 5w.t.% hypromellose aqueous solution, then add polyethylene glycol 6000, titanium dioxide, and talcum powder, and stir evenly. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com