Method for in situ preparing of all-solid-state electrolyte
An in-situ preparation and electrolyte technology, applied in composite electrolytes, nanotechnology for materials and surface science, circuits, etc., can solve problems such as failure to enhance ionic conductivity, easy agglomeration, etc. Good flexibility and the effect of improving ionic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 0.75g of PEO to 15ml of anhydrous acetonitrile and stir to form a uniform solution, then add 1mL of deionized water, 60mL of absolute ethanol, 3mL of ammonia water, and 2.3mL of ethyl orthosilicate in sequence, and stir at 20°C for 6h to obtain a uniform solution. of white turbid liquid. The obtained white cloudy liquid was evaporated to dryness by a rotary evaporator. Add 15ml of anhydrous acetonitrile, magnetically stir until a uniform white turbid liquid is added, then add 0.165g LiBOB and stir for 24 hours, then pour it into a polytetrafluoroethylene container, and evaporate the solvent in a vacuum oven at 40°C to obtain a uniform film. The membrane was placed in the glove box for 48h to obtain the final solid electrolyte.
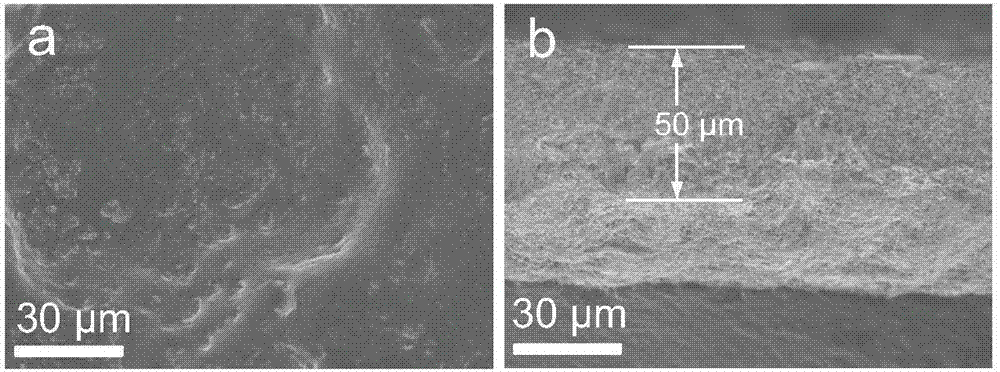
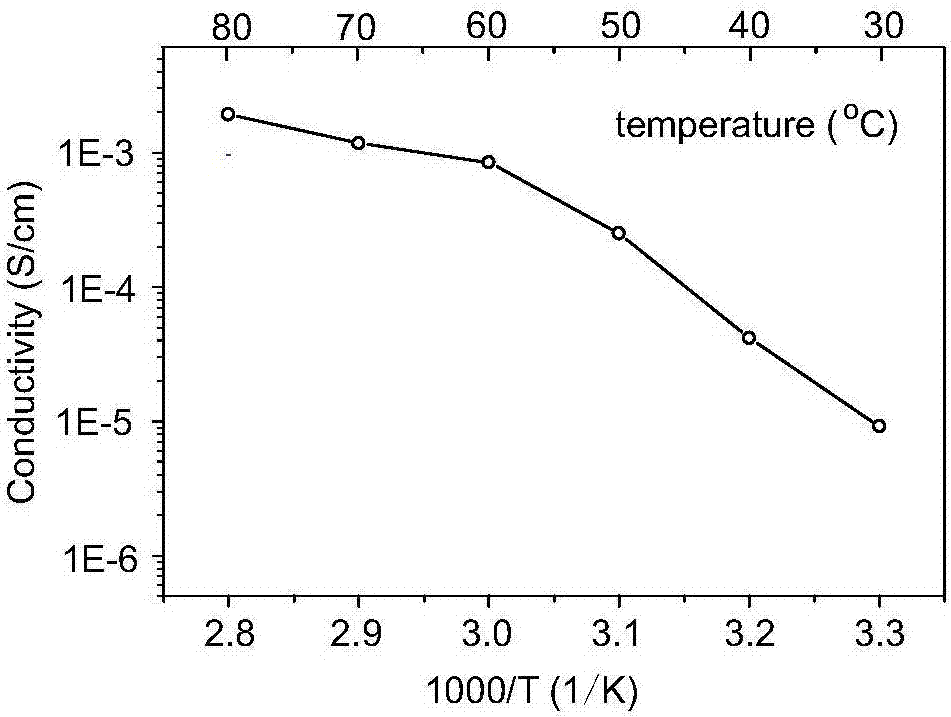
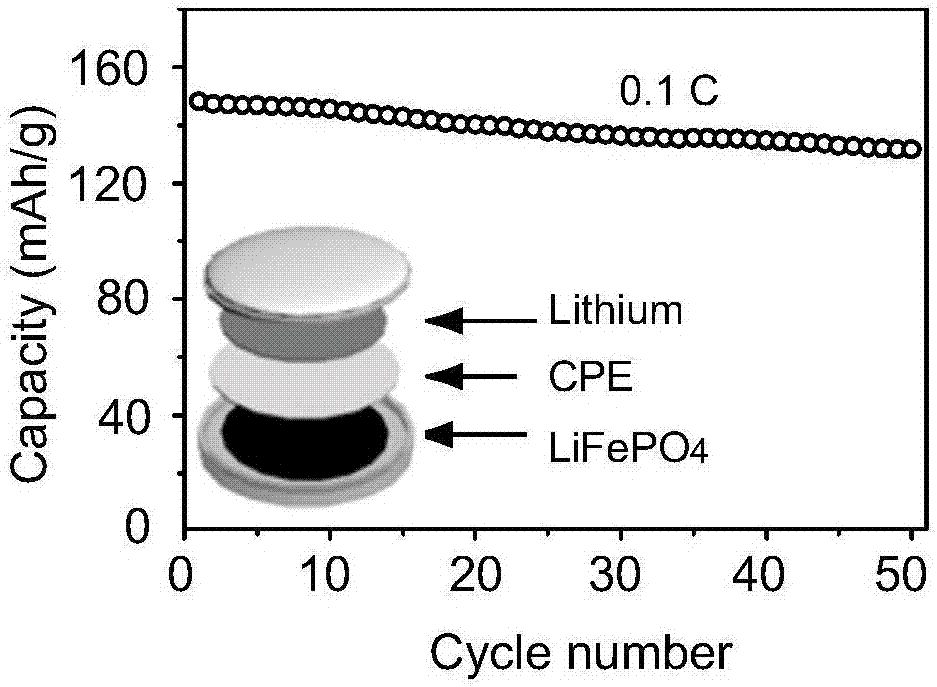
[0033] The morphology of the solid electrolyte prepared in situ in this example was tested, figure 1 It is a SEM picture, it can be seen from the picture that SiO 2 The nanoparticles are relatively uniformly distributed in the PEO framewo...
Embodiment 2
[0037]Add 0.75g of PEO to 15ml of anhydrous acetonitrile and stir to form a uniform solution, then add 1mL of deionized water, 60mL of absolute ethanol, 3mL of ammonia water, 0.5mL of ethyl orthosilicate, and stir at 20°C for 6h to obtain a uniform solution. of white turbid liquid. The obtained white cloudy liquid was evaporated to dryness by a rotary evaporator. Add 15ml of anhydrous acetonitrile, stir magnetically until a uniform white turbid liquid is obtained, then add 0.165g LiBOB and stir for 24 hours, then pour it into a polytetrafluoroethylene container, evaporate the solvent to dryness in a vacuum oven at 40°C to obtain a uniform film, and then The final solid electrolyte was obtained by placing the film in the glove box for 48 hours.
[0038] The morphology of the solid electrolyte prepared in situ in this example was tested, and it can be seen from the SEM image that SiO 2 Nanoparticles are relatively uniformly distributed in the PEO framework, and we also measure...
Embodiment 3
[0041] Add 0.75g of PEO to 15ml of anhydrous acetonitrile and stir to form a homogeneous solution, then add 1mL of deionized water, 60mL of absolute ethanol, 3mL of ammonia water, 1mL of ethyl orthosilicate, and stir at 20°C for 6h to obtain a uniform solution. White cloudy liquid. The obtained white cloudy liquid was evaporated to dryness by a rotary evaporator. Add 15ml of anhydrous acetonitrile, stir magnetically until a uniform white turbid liquid is obtained, then add 0.165g LiBOB and stir for 24 hours, then pour it into a polytetrafluoroethylene container, evaporate the solvent to dryness in a vacuum oven at 40°C to obtain a uniform film, and then The final solid electrolyte was obtained by placing the film in the glove box for 48 hours.
[0042] The morphology of the solid electrolyte prepared in situ in this example was tested, and it can be seen from the SEM image that SiO 2 Nanoparticles are relatively uniformly distributed in the PEO framework, and we also measure...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com