Sulfonated hydroxypropyl chitosan modified biocompatible polysulfone membrane and preparation method thereof
A technology of sulfonated hydroxypropyl chitosan and sulfonated hydroxypropyl shell, which is applied in the field of sulfonated hydroxypropyl chitosan modified polysulfone membrane and its preparation, and can solve complicated processes, deformation, platelet adsorption, etc. problems, to achieve the effects of mild and easy control of reaction conditions, low cost of raw materials, and improved stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
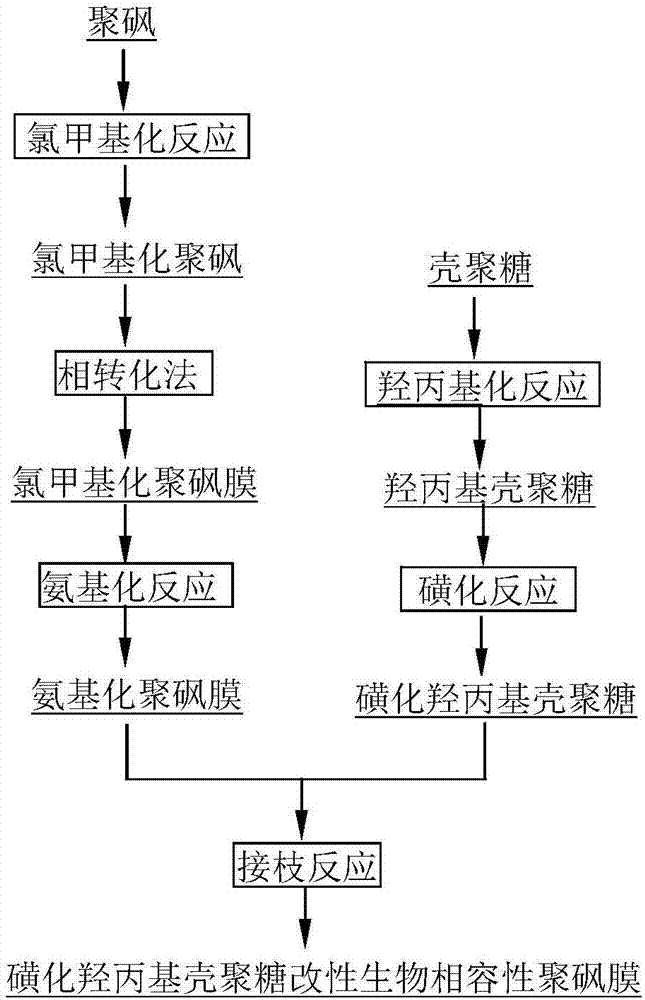
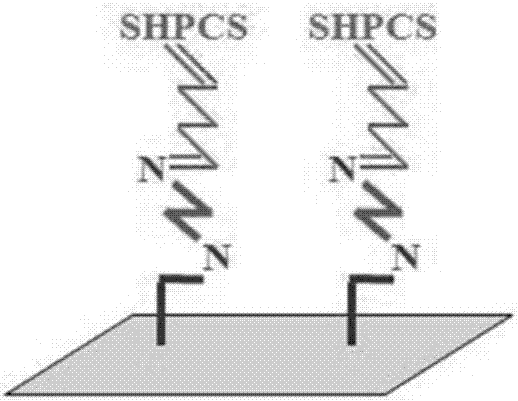
[0049] Take 15g CS as raw material, first alkalinize it with NaOH solution with a concentration of 10moL / L, add it into the reactor together with 150ml IPA and 150ml PO, stir and react at 45°C for 4h, then pour it into deionized water to dissolve and neutralize , followed by acetone precipitation, suction filtration, washing and drying to obtain HPCS. Mix 2ml of CSA and 20ml of formamide in an ice-salt bath to make a sulfonation reagent, then add 2g of HPCS, stir and react at 68°C for 3h, and undergo dialysis, deamination, redialysis, concentration and drying to obtain SHPCS.
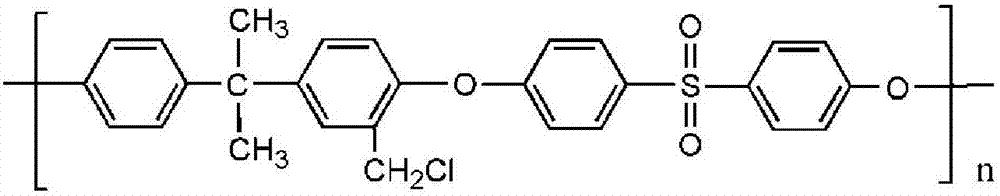
[0050] Add 250ml chloroform to the flask, add 10g PSf and dissolve, add 28.5ml TMCS, 6.7g PFA and 0.26ml SnCl 4 , stirred at 45°C under nitrogen protection for 72h, precipitated with methanol, vacuum filtered and dried to obtain PSf-Cl. Then dissolve PSf-Cl in N,N-dimethylacetamide with a mass fraction of 18%, configure it as a casting solution, first shake it to dissolve, then let it stand for defoami...
Embodiment 2
[0053] Take 15g CS as raw material, first alkalinize it with NaOH solution with a concentration of 10moL / L, add it into the reactor together with 150ml IPA and 150ml PO, stir and react at 45°C for 4h, then pour it into deionized water to dissolve and neutralize , followed by acetone precipitation, suction filtration, washing and drying to obtain HPCS. 4ml of CSA and 20ml of formamide were formulated into a sulfonation reagent in an ice-salt bath, then 2g of HPCS was added, the reaction was stirred at 68°C for 3h, and SHPCS was obtained by dialysis, deamination, redialysis, and concentration and drying.
[0054] Add 250ml chloroform to the flask, add 10g PSf and dissolve, add 28.5ml TMCS, 6.7g PFA and 0.26ml SnCl 4 , stirred at 45°C under nitrogen protection for 72h, precipitated with methanol, vacuum filtered and dried to obtain PSf-Cl. Then dissolve PSf-Cl in N,N-dimethylacetamide with a mass fraction of 18%, configure it as a casting solution, first shake it to dissolve, th...
Embodiment 3
[0057] Take 15g CS as raw material, first alkalinize it with NaOH solution with a concentration of 10moL / L, add it into the reactor together with 150ml IPA and 150ml PO, stir and react at 45°C for 4h, then pour it into deionized water to dissolve and neutralize , followed by acetone precipitation, suction filtration, washing and drying to obtain HPCS. 4ml of CSA and 20ml of formamide were formulated into a sulfonation reagent in an ice-salt bath, then 2g of HPCS was added, the reaction was stirred at 68°C for 3h, and SHPCS was obtained by dialysis, deamination, redialysis, and concentration and drying.
[0058] Add 250ml chloroform to the flask, add 10g PSf and dissolve, add 28.5ml TMCS, 6.7g PFA and 0.26ml SnCl 4 , stirred at 60°C under nitrogen protection for 72h, precipitated with methanol, vacuum filtered and dried to obtain PSf-Cl. Then dissolve PSf-Cl in N,N-dimethylacetamide with a mass fraction of 18%, configure it as a casting solution, first shake it to dissolve, th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com