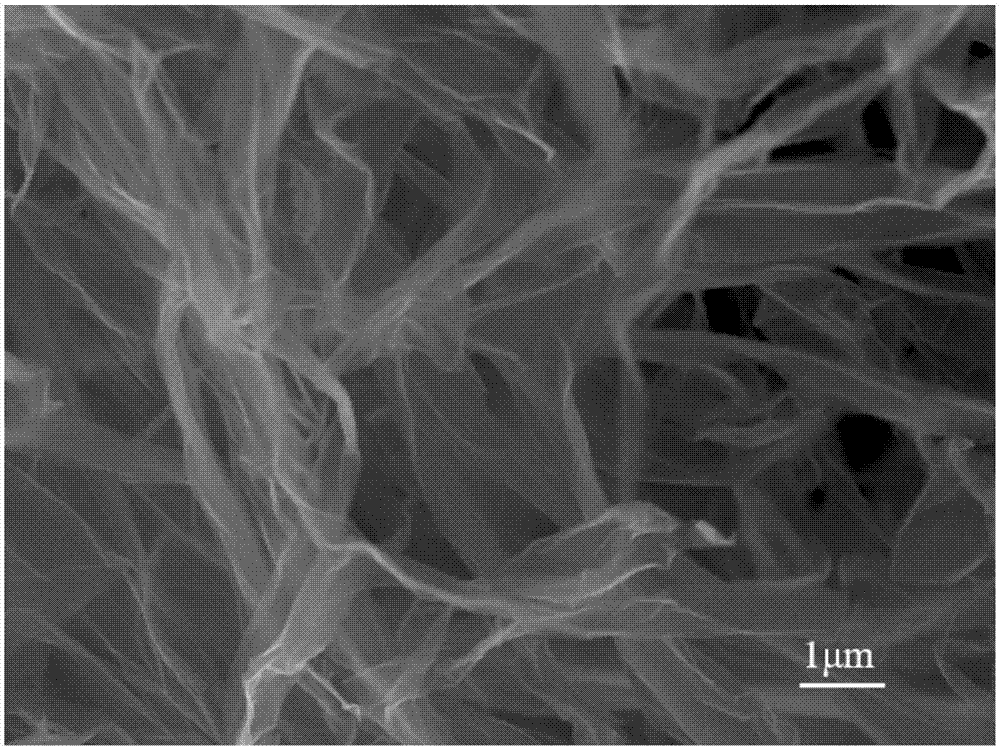
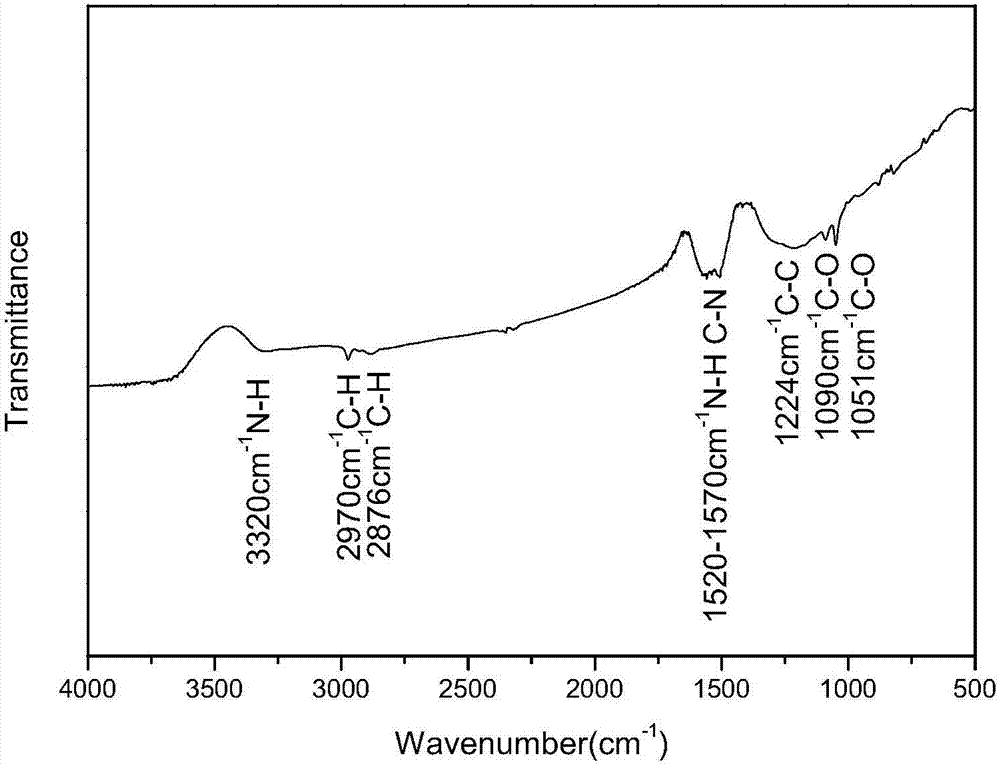
Preparation method of aminated graphene
A technology of aminated graphene and carboxylated graphene, applied in chemical instruments and methods, inorganic chemistry, carbon compounds, etc., can solve problems such as physical health damage, environmental damage, and low amino content, and prevent structural changes. , the effect of avoiding agglomeration and preventing damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Preparation of graphene oxide
[0021] The method for synthesizing graphene oxide in the present invention is a chemical oxidation method.
[0022] The source of the amino group in the present invention is the carboxyl group in the graphene oxide, so the carboxyl group content in the graphene oxide directly affects the doping amount of the amino group in the aminated graphene. Therefore, in the process of synthesizing graphene oxide, we changed the concentrated sulfuric acid into a mixed acid of concentrated sulfuric acid and concentrated phosphoric acid and prolonged the oxidation time to increase the oxidation degree of graphite and increase the reactive sites. The volume ratio of concentrated sulfuric acid to concentrated phosphoric acid is 1:1, and the oxidation time is 2 hours.
[0023] (2) Preparation of Aminated Graphene
[0024] Disperse graphene oxide uniformly in aqueous solution with a concentration of 1mol L -1 , then add NaOH and chloroacetic acid wi...
Embodiment 2
[0030] (1) Preparation of graphene oxide
[0031] The method for synthesizing graphene oxide in the present invention is a chemical oxidation method.
[0032] The source of the amino group in the present invention is the carboxyl group in the graphene oxide, so the carboxyl group content in the graphene oxide directly affects the doping amount of the amino group in the aminated graphene. Therefore, in the process of synthesizing graphene oxide, we changed the concentrated sulfuric acid into a mixed acid of concentrated sulfuric acid and concentrated phosphoric acid and prolonged the oxidation time to increase the oxidation degree of graphite and increase the reactive sites. The volume ratio of concentrated sulfuric acid to concentrated phosphoric acid is 3:1, and the oxidation time is 5 hours.
[0033] (2) Preparation of Aminated Graphene
[0034] Disperse graphene oxide uniformly in aqueous solution with a concentration of 0.5mol L -1 , then add NaOH and chloroacetic acid ...
Embodiment 3
[0040] (1) Preparation of graphene oxide
[0041] The method for synthesizing graphene oxide in the present invention is a chemical oxidation method.
[0042] The source of the amino group in the present invention is the carboxyl group in the graphene oxide, so the carboxyl group content in the graphene oxide directly affects the doping amount of the amino group in the aminated graphene. Therefore, in the process of synthesizing graphene oxide, we changed the concentrated sulfuric acid into a mixed acid of concentrated sulfuric acid and concentrated phosphoric acid and prolonged the oxidation time to increase the oxidation degree of graphite and increase the reactive sites. The volume ratio of concentrated sulfuric acid to concentrated phosphoric acid is 8:1, and the oxidation time is 3 hours.
[0043] (2) Preparation of Aminated Graphene
[0044] Disperse graphene oxide uniformly in aqueous solution with a concentration of 2mol L -1, then add NaOH and chloroacetic acid wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com