Combined medication combination for tumor therapy
A tumor treatment and combination drug technology, applied in the biological field, can solve the problem of not disclosing the relationship between ACAT1 and tumor treatment, and achieve the effect of an effective treatment plan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
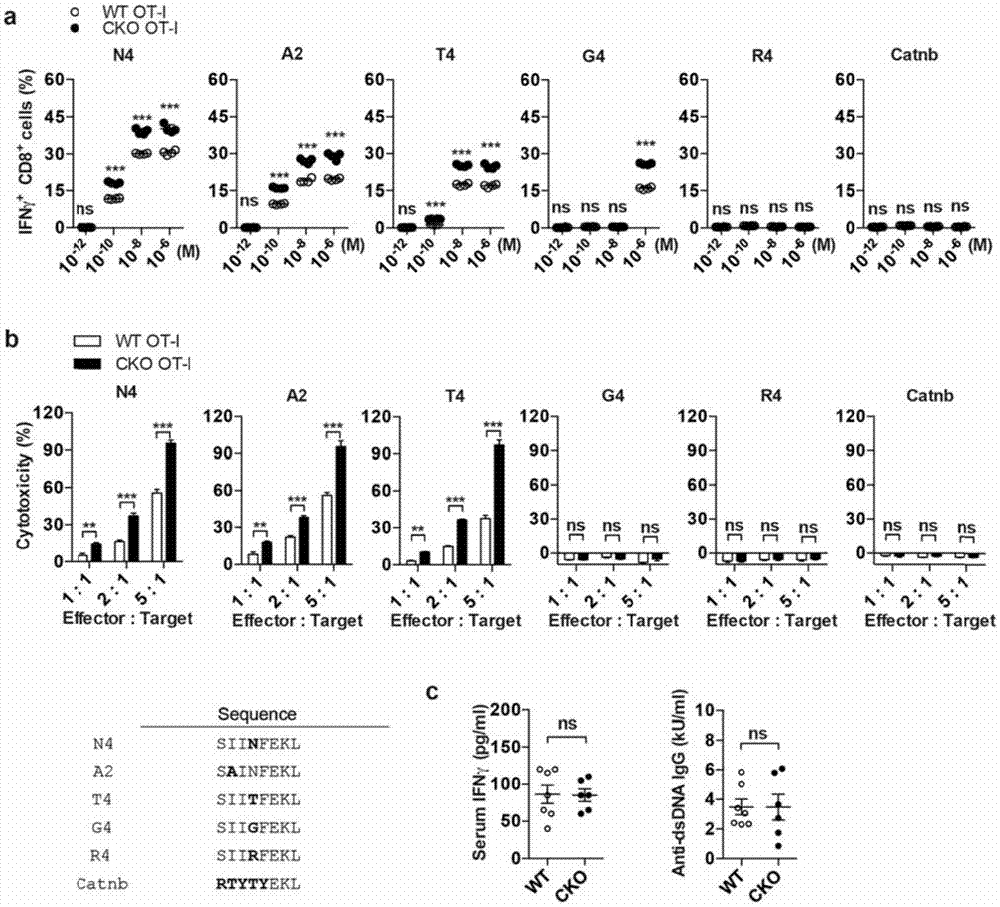
[0199] Example 1 ACAT1 gene knockout does not trigger CD8 T cells to respond to self-antigens and autoimmunity
[0200] The purpose of this example is to study whether the enhanced effector function of CD8 T cells after removing the function of ACAT1 will make CD8 T cells respond to self-antigens and induce autoimmune diseases. To study this, the Acat1 CKO The mice were further mated with OT-I TCR transgenic mice (Jax mice) to obtain ACAT1-specific knockout mice (OT-I Acat1 CKO ) and corresponding control mice (OT-I). The vast majority of T cells in OT-I transgenic mice are OT-I CD8 T cells, whose TCR specifically recognizes H2K b Presented Ovalbumin (OVA 257-264 ) antigen (N4 for short), and generate the corresponding CD8 T cell immune response; at the same time, OT-I TCR can also recognize a series of OVA mutants (A2, T4, G4, R4) in addition to its wild-type antigen (N4). ), and generate a corresponding strong or weak immune response. Experiments have found that ACAT1 d...
Embodiment 2
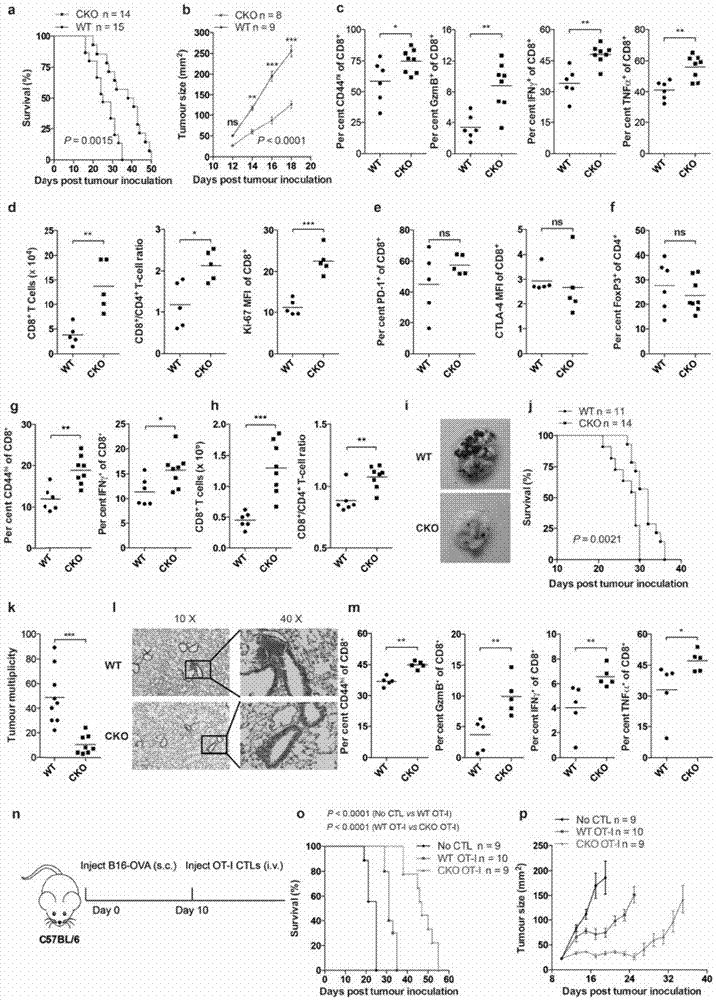
[0201] Example 2 Using Three Melanoma Models to Study ACAT1 Gene Knockout Promotes the Anti-tumor Activity of CD8 T Cells
[0202] Immunotherapy is currently a hot spot in the treatment of tumors, and the ability of CD8 T cells to kill tumor cells is directly related to the effect of tumor immunotherapy. In order to verify the effect of ACAT1 knockout on the anti-tumor ability of CD8 T cells, this example first established a subcutaneous melanoma model, in which Acat1 CKO Melanoma induced by subcutaneous injection of B16F10 melanoma cells in mice and wild-type mice, Acat1 CKO The progression of melanoma in mice was significantly delayed, as demonstrated by slower tumor growth and longer survival and survival in mice ( figure 2 .a,b). The phenotype of T cells in tumor-bearing mice was analyzed, and it was found that Acat1 CKO In mice, tumor-infiltrating CD8 T cells were more active and expressed Acat1 CKO In tumor-infiltrating CD8 T cells in mice, there are more effector c...
Embodiment 3
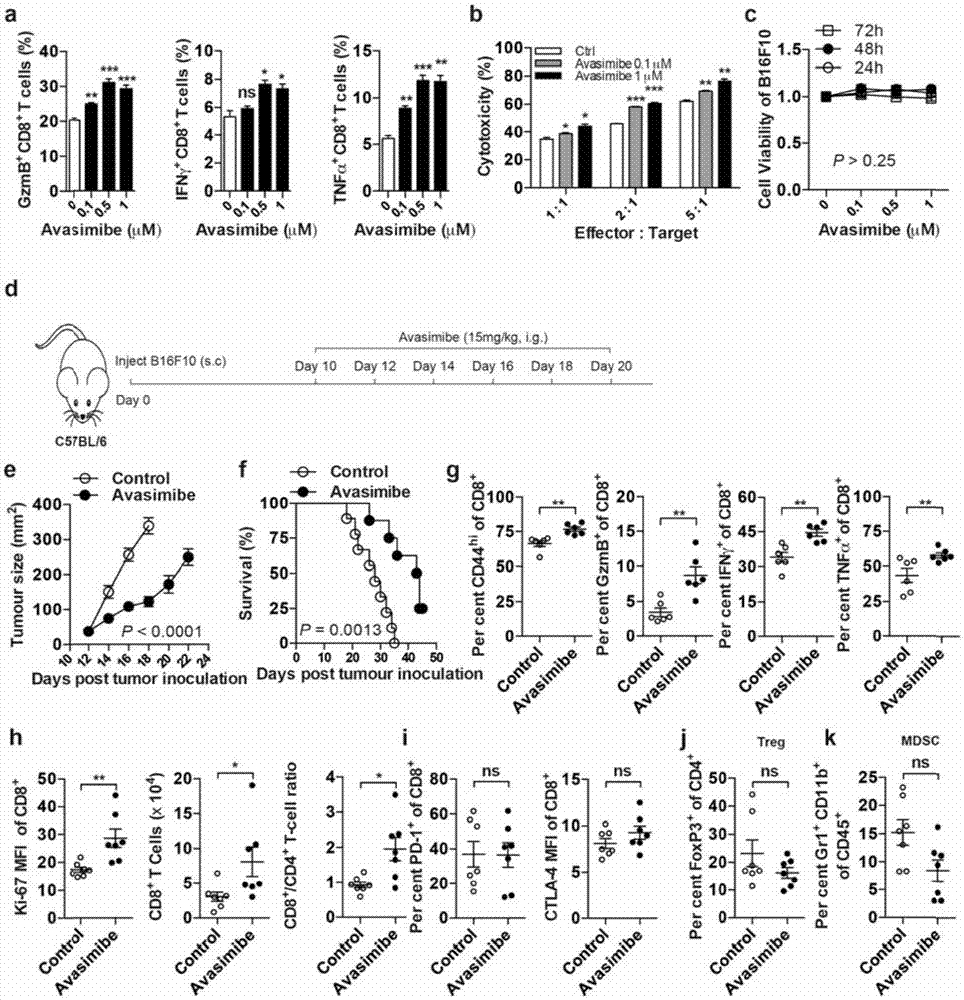
[0206] Example 3 ACAT inhibitor Avasimibe enhances the anti-tumor function of CD8 T cells.
[0207] The purpose of this example is to further verify the application of ACAT1 inhibitors in tumor immunotherapy. ACAT1 is used as a target for the treatment of atherosclerosis, and a series of small molecule drugs are currently undergoing animal and clinical trials. Avasimibe is one of them, and many clinical trials have verified the safety of Avasimibe. Therefore, Avasimibe was selected to verify the possibility of ACAT1 as a target for tumor immunotherapy. In vitro experiments showed that Avasimibe, like other ACAT inhibitors such as CP113, 818 and K604, could promote the release of granzyme B and the expression of cytokines IFNγ and TNFα in CD8 T cells ( image 3 .a). The in vitro killing experiment also showed that Avasimibe enhanced the target cell killing ability of CTL, and showed a dose effect ( image 3 .b). The experiment further verified the effect of Avasimibe in th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com