Method for hydrolyzing isopropyl nitrate
A technology of isopropyl nitrate and aqueous solution, applied in directions such as hydrolysis preparation, organic chemistry, etc., can solve problems such as reducing efficiency, waste, shortening, etc., and achieve the effects of simple experimental conditions, high deesterification rate, and short time consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
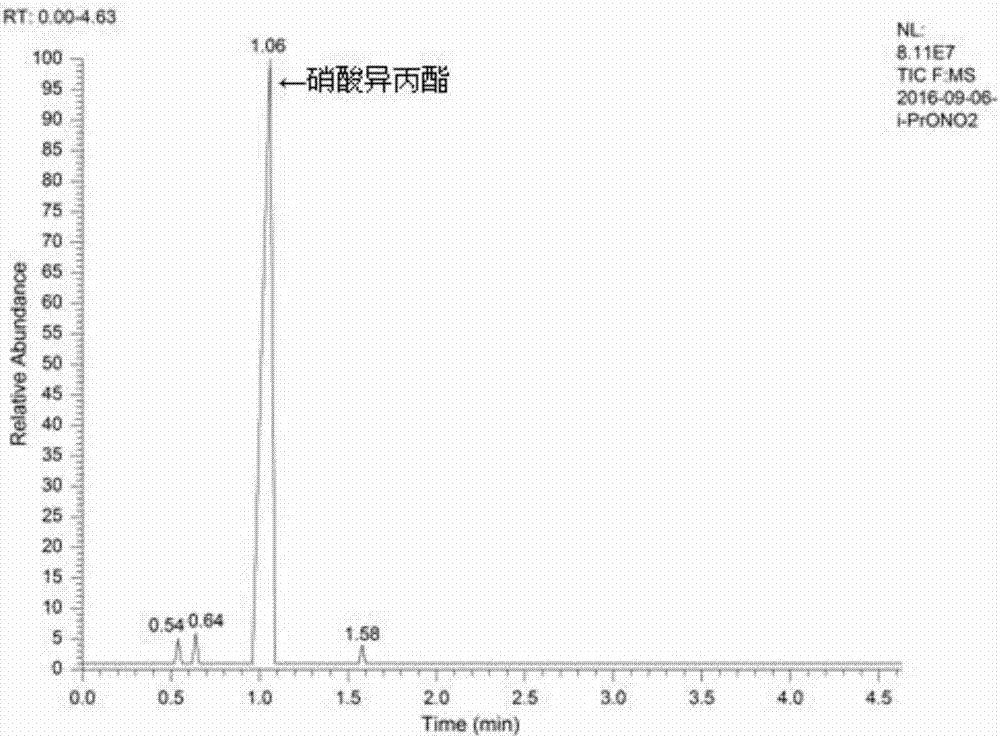
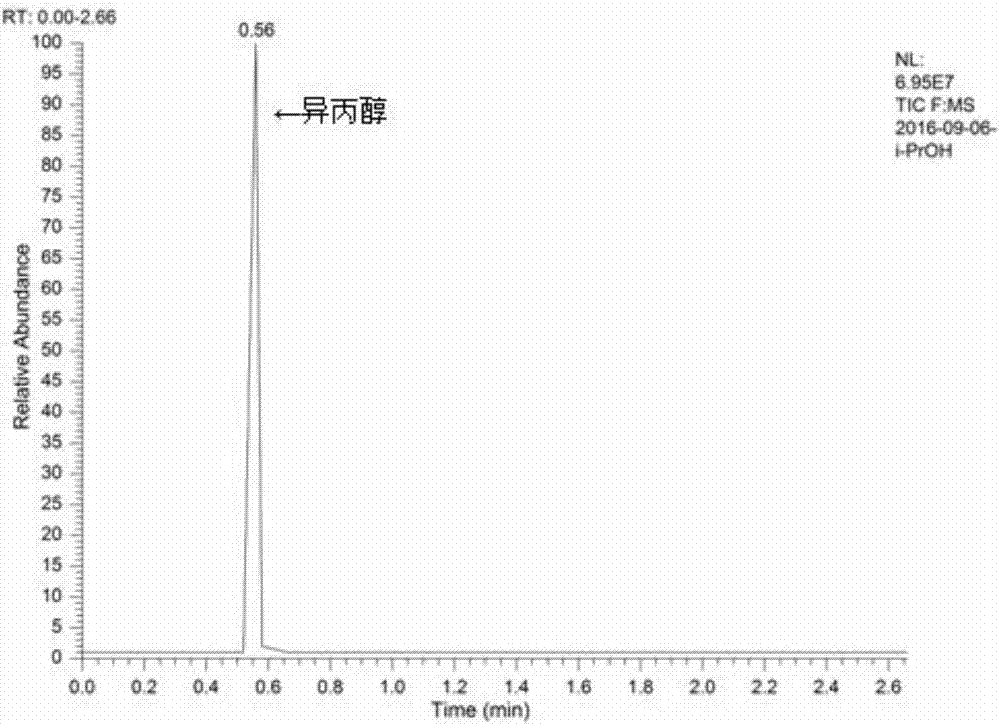
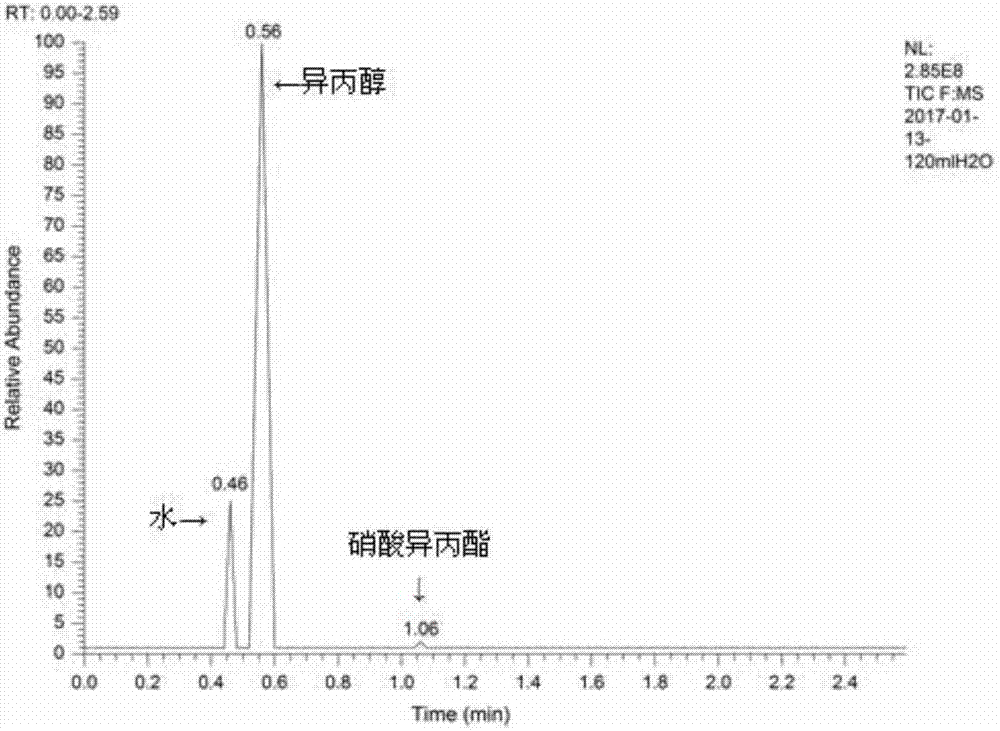
[0016] Example 1: Add isopropyl nitrate (20mL, 0.2mol), sodium hydroxide (6.4g, 0.16mol), water (40mL, 2.22mol) into the autoclave, heat to 140°C in the autoclave, and the pressure is 0.9MPa , start to react, after reacting for 30 minutes, cool down to room temperature, distill the reaction solution, collect fractions at 80-84°C, gas detection, the results are as attached image 3 shown, with the attached figure 1 It can be seen that the raw material isopropyl nitrate reacts almost completely, and with the attached figure 2 It can be seen that the peak at the retention time of 0.56 min is the isopropanol peak. The calculated removal rate of isopropyl nitrate is 98.4%, and the yield of isopropanol is 66.8%.
Embodiment 2
[0017] Example 2: Add isopropyl nitrate (20mL, 0.2mol), sodium hydroxide (8.8g, 0.22mol), water (80mL, 4.44mol) into the autoclave, heat to 140°C in the autoclave, and the pressure is 0.8MPa , start to react, after reacting for 30 minutes, cool down to room temperature, distill the reaction solution, collect fractions at 80-84°C, gas detection, the results are as attached image 3 shown, with the attached figure 1 It can be seen that the raw material isopropyl nitrate reacts almost completely, and with the attached figure 2 It can be seen that the peak at the retention time of 0.56 min is the isopropanol peak. The calculated removal rate of isopropyl nitrate is 99.1%, and the yield of isopropanol is 63.6%.
Embodiment 3
[0018] Example 3: Add isopropyl nitrate (20mL, 0.2mol), sodium hydroxide (12g, 0.3mol), water (100mL, 5.56mol) into the autoclave, heat to 140°C in the kettle, pressure 0.8MPa, Start the reaction, after reacting for 30 minutes, cool down to room temperature, distill the reaction solution, collect fractions at 80-84°C, and test the gaseous quality. The results are shown in the attached image 3 shown, with the attached figure 1 It can be seen that the raw material isopropyl nitrate reacts almost completely, and with the attached figure 2 It can be seen that the peak at the retention time of 0.56 min is the isopropanol peak. The calculated removal rate of isopropyl nitrate is 99.7%, and the yield of isopropanol is 64.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com