Method for simply synthesizing pyraclostrobin
A pyraclostrobin, a concise technology, applied in the field of organic compound synthesis, can solve problems such as potential safety hazards, many reaction by-products, etc., and achieve the effects of promoting methylation speed, simplifying experimental conditions, and avoiding hydrolysis by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] This embodiment relates to a simple method for synthesizing pyraclostrobin, the content is as follows:
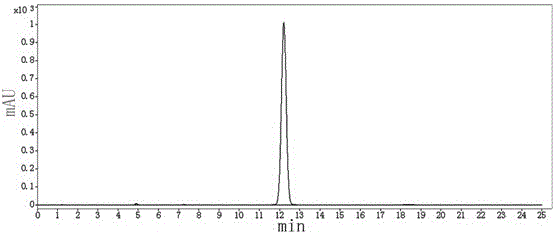
[0045] Put 330kg of pyraclostrobin intermediate and 8.25kg of phase transfer catalyst (benzyltriethylammonium chloride) into a 3000L reaction kettle, and then put 2310kg of organic solvent (methyl tert-butyl ether ) to dissolve, start stirring and freezing the saline solution to maintain the temperature of the system at 45°C, then put 121kg of dimethyl sulfate into the reactor, and add 350kg of 12% sodium hydroxide aqueous solution dropwise at this temperature, and the dropping time is controlled at After 60 minutes, after the dropwise addition was completed and incubated for 2 hours, samples were taken, and the reaction was stopped after HPLC monitoring until the peak area percentage of the pyraclostrobin intermediate was less than 0.5%.
[0046] After the reaction is completed, heat the steam to 60°C and keep it warm for 3 hours. After the heat preservation is comp...
Embodiment 2
[0049] This embodiment relates to a simple method for synthesizing pyraclostrobin, the content is as follows:
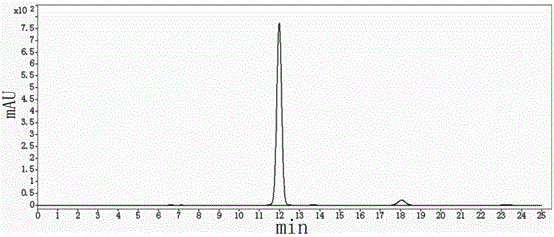
[0050] Put 350kg of pyraclostrobin intermediate and 7kg of phase transfer catalyst (benzyltriethylammonium chloride) into a 3000L reaction kettle, then put 2800kg of organic solvent (chloroform) from the storage tank to dissolve, and start stirring Keep the temperature of the system at 20°C with frozen saline solution, then put 130kg of dimethyl sulfate into the reaction kettle, and add 560kg of 8% sodium hydroxide aqueous solution dropwise at this temperature, the dropping time is controlled at 30 minutes, and the dropping is completed Samples were taken after incubation for 2 hours, and the reaction was stopped after HPLC monitoring until the peak area percentage of the pyraclostrobin intermediate was less than 0.5%.
[0051] After the reaction is completed, heat the steam to 65°C and keep it warm for 3 hours. After the heat preservation is completed, let it stand ...
Embodiment 3
[0054] This example relates to a method for concisely synthesizing pyraclostrobin, the content is as follows:
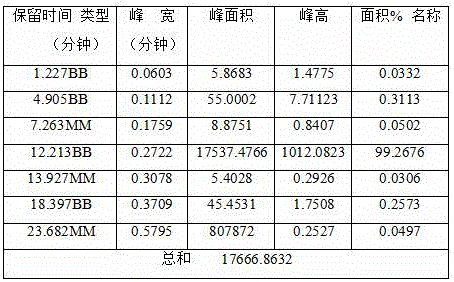
[0055] Put 365kg of pyraclostrobin intermediate and 16.5kg of phase transfer catalyst (tetraethylammonium bromide, tetrabutylammonium chloride and tetrabutylammonium bromide) into a 3000L reactor, and then from the storage tank Put 2920kg of organic solvent (dichloromethane) to dissolve, start stirring and freezing the saline solution to maintain the temperature of the system at 15°C, then put 135kg of dimethyl sulfate into the reaction kettle, and add 12% hydroxide dropwise at this temperature 390g of sodium aqueous solution, the dropping time is controlled at 50 minutes, after the dropping is completed and kept warm for 2 hours, the sample is taken, and the reaction is stopped after HPLC monitoring until the peak area percentage of the pyraclostrobin intermediate is less than 0.5%.
[0056] After the reaction is completed, heat the steam to 60°C and keep it warm fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com