Improved method of synthesizing 4-chlorine-1hydrogen-quinoline-2-ketone-3-carboxylic methyl ester by isatoic anhydride
A technology of methyl carboxylate and isatoic anhydride, which is applied in the field of chemical synthesis, can solve the problems of large consumption of organic solvents, and achieve the effects of less solvent residue, high yield, and simple purification process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
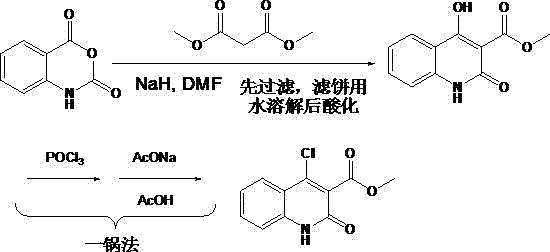
[0012] Embodiment 1: Preparation of 4-hydroxyl-1 hydrogen-quinolin-2-one-3-carboxylic acid methyl ester
[0013] Dissolve NaH (60%) (0.80g, 20.0mmoL) in 30mL DMF in a 50mL three-neck flask equipped with magnetic stirring, and add dimethyl malonate (2.43 g, 18.2mmoL) dropwise at -10°C, After dropping, the temperature was raised to room temperature. Stir at room temperature for 20min, add isatoic anhydride (3.00g, 18.2mmoL) to the above mixture at one time, stop heating and stirring after reacting at 110°C for 1h, cool to room temperature and collect the solid by suction filtration. Dissolve the solid in 15mL water, acidify with hydrochloric acid at low temperature to pH ≈ 4, a large amount of white solid is produced, filter the filter cake obtained by suction and dry to obtain methyl 4-hydroxy-1hydro-quinolin-2-one-3-carboxylate Pure ester (3.73g, 92.5% yield).
Embodiment 2
[0014] Embodiment 2: Preparation of 4-hydroxyl-1 hydrogen-quinolin-2-one-3-carboxylic acid methyl ester
[0015] Dissolve NaH (60%) (8.00 g, 0.200 moL) in 300 mL of DMF in a 500 mL three-neck flask equipped with mechanical stirring, and add dimethyl malonate (24.3 g, 0.182 moL) dropwise at -10 °C, After dropping, the temperature was raised to room temperature. Stir at room temperature for 20min, add isatoic anhydride (30.0g, 0.182moL) to the above mixture at one time, stop heating and stirring after reacting at 110°C for 1h, cool to room temperature and collect the solid by suction filtration. Dissolve the solid in 110mL of water, acidify with hydrochloric acid at low temperature to pH ≈ 4, a large amount of white solid is produced, filter the filter cake obtained by suction and dry to obtain methyl 4-hydroxy-1hydro-quinolin-2-one-3-carboxylate Pure ester (38.3 g, 96% yield).
Embodiment 3
[0016] Embodiment 3: Preparation of 4-hydroxyl-1 hydrogen-quinolin-2-one-3-carboxylic acid methyl ester
[0017] The feeding amount and feeding method are the same as in Example 2. The post-treatment method adopts the previously reported method. The reaction solution is cooled to room temperature, poured into 2L of ice water, and hydrochloric acid is added at a low temperature. Only a small amount of solids are produced when acidifying to pH ≈ 4. , when acidified to pH ≈ 2, a large amount of solids were produced, and 28.2 g of the product was obtained after suction filtration, washing and drying, with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com