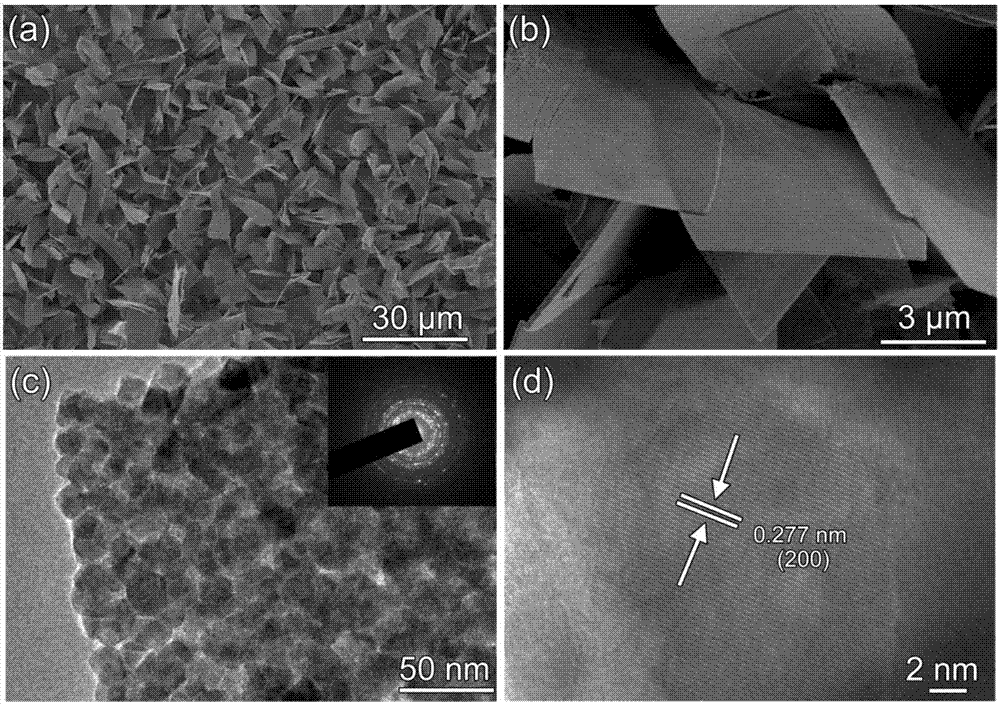
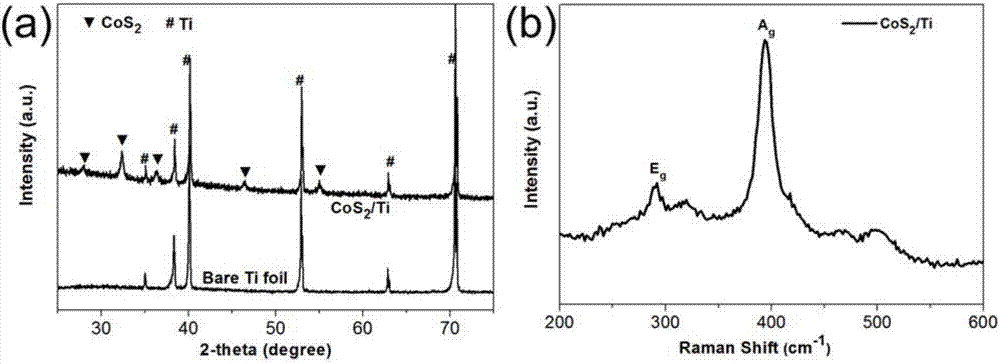
Synthetic method of cobalt disulfide nanoribbon assembled structure on titanium substrate
A technology of cobalt disulfide and assembly structure, which is applied in chemical instruments and methods, electrolytic components, chemical/physical processes, etc., can solve the problem that the catalytic hydrogen production performance of pure phase cobalt disulfide cannot be further improved, the product is not uniform, and the In order to achieve the effect of promoting charge transfer, lowering the reaction barrier, and strong controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Co(OH) on titanium substrate 3 Synthesis of nanoribbons
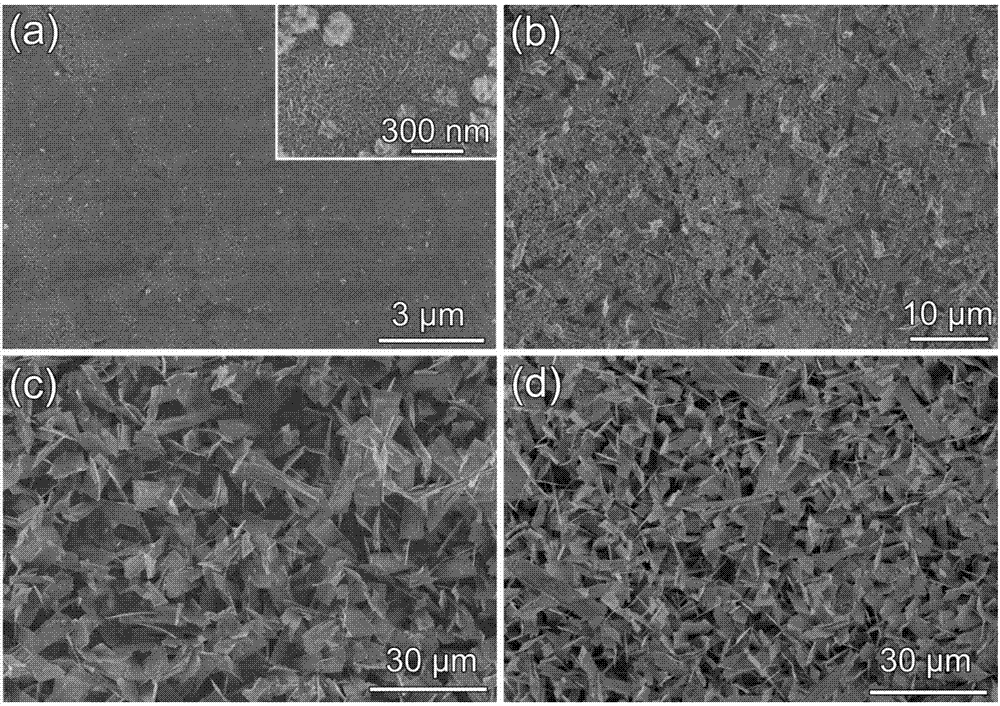
[0032] Weigh 0.0065g cobalt dichloride hexahydrate and 0.0125g cetyltrimethylammonium chloride (CTAC), dissolve in 5mL water and 5mL N, N-dimethylformamide (DMF) mixed solution, gradually Add 2mL of hydrogen peroxide (30%) solution dropwise, transfer to a 50mL reaction kettle, put in the treated clean bare titanium sheet, package, place it at 150°C for 1 hour, take out the titanium sheet, and rinse with ethanol and deionized water in turn Clean, dry at 60°C to get self-assembled Co(OH) 3nanobelt. The samples obtained were figure 1 As shown in (a), the scanning electron microscope shows that when the reaction time is 1h, the surface of the titanium substrate is a dendritic material, which has not yet grown into a nanoribbon morphology, indicating that Co(OH) 3 Indeed, in situ nucleation occurs on the surface of the titanium substrate and in situ growth begins.
Embodiment 2
[0034] Co(OH) on titanium substrate 3 Synthesis of nanoribbons
[0035] Weigh 0.0065g cobalt dichloride hexahydrate and 0.0125g cetyltrimethylammonium chloride (CTAC), dissolve in 5mL water and 5mL N, N-dimethylformamide (DMF) mixed solution, gradually Add 2mL of hydrogen peroxide (30%) solution dropwise, transfer to a 50mL reactor, put in the treated clean bare titanium sheet, package, place it at 150°C for 5 hours, take out the titanium sheet, and rinse with ethanol and deionized water in turn Clean, dry at 60°C to get self-assembled Co(OH) 3 nanobelt. The samples obtained were figure 1 As shown in (b), the scanning electron microscope shows that when the reaction time is 5h, Co(OH) with a length of about 3 microns and a width of about 1 micron has appeared on the surface of the titanium substrate. 3 Nanobelts, illustrating Co(OH) 3 It has grown from dendrites in 1h to the morphology of small nanoribbons.
Embodiment 3
[0037] Co(OH) on titanium substrate 3 Synthesis of nanoribbons
[0038] Weigh 0.0065g cobalt dichloride hexahydrate and 0.0125g cetyltrimethylammonium chloride (CTAC), dissolve in 5mL water and 5mL N, N-dimethylformamide (DMF) mixed solution, gradually Add 2mL of hydrogen peroxide (30%) solution dropwise, transfer to a 50mL reaction kettle, put in the treated clean bare titanium sheet, package, place it at 150°C for 10 hours, take out the titanium sheet, and rinse with ethanol and deionized water in turn Clean, dry at 60°C to get self-assembled Co(OH) 3 nanobelt. The samples obtained were figure 1 As shown in (c), SEM shows that when the reaction time is 10h, Co(OH) 3 The nanobelts have been evenly distributed on the surface of the titanium substrate, the structure is stable, and the substrate is covered with the substrate, and the size has further increased compared with 5h.
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com