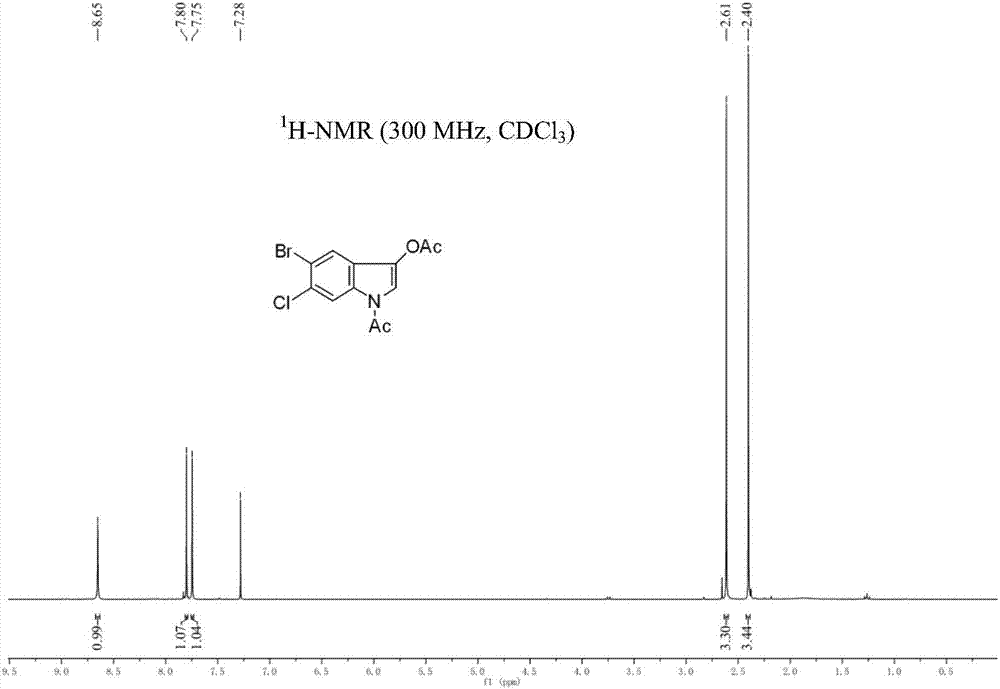
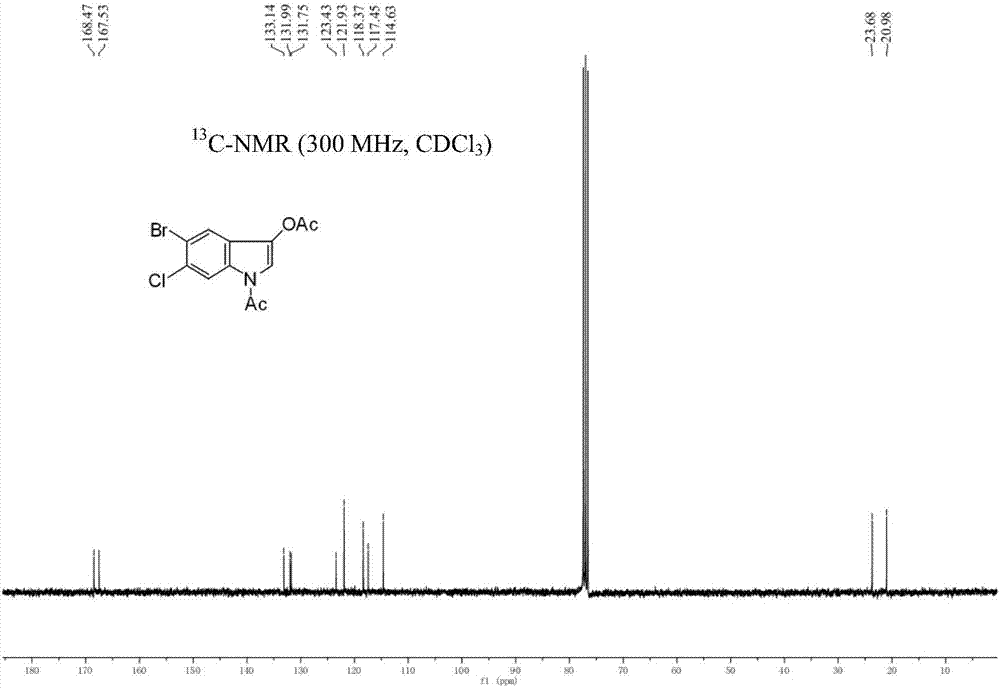
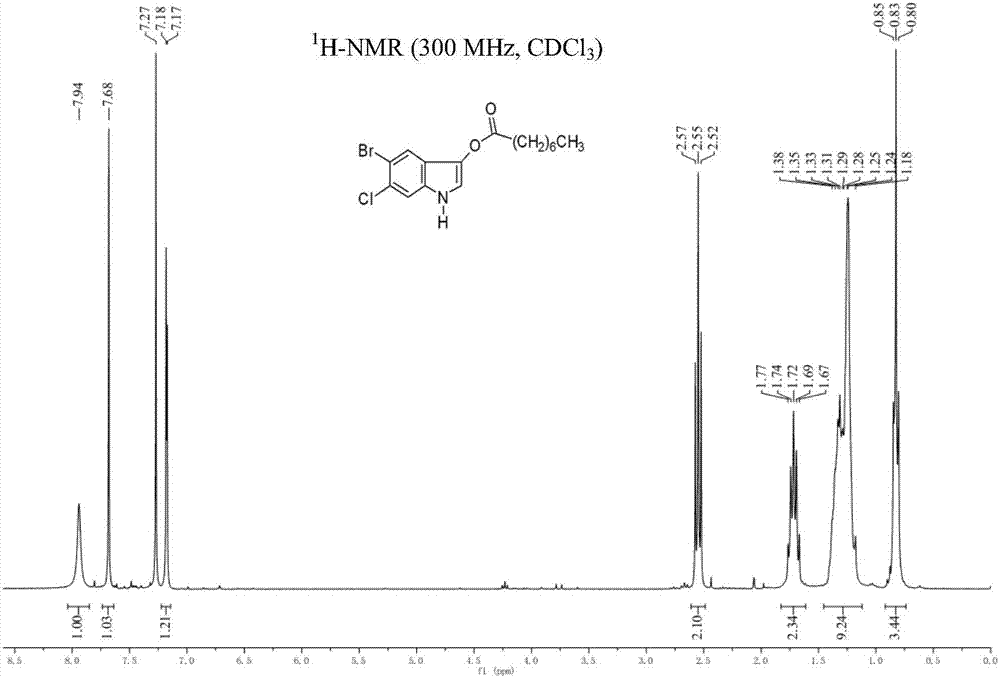
5-bromo-6-chloro-3-indolyl caprylate synthesis method
A technology for indole octyl ester and a synthesis method, which is applied in the fields of organic synthesis and biological analysis and detection, can solve the problems of many pollutants, low total yield, difficult recovery and the like, and achieves the effect of high reaction total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The synthetic route of the present embodiment is shown in the following formula:
[0030]
[0031] (1) Synthesis of 5-bromo-4-chloro-2-aminobenzoic acid (Ⅱ)
[0032] Add 4-chloro-2-aminobenzoic acid (I; 20.00g, 116.6mmol) into a 500mL single-necked round-bottomed flask, then add acetonitrile (300mL), and stir rapidly to form a beige suspension. N-bromosuccinimide (NBS; 20.75g, 116.6mmol) was added slowly, and the stirring reaction was continued for 1h after the addition was completed, then the solvent was removed by rotary evaporation in a water bath at 45°C, and water was added to stir, suction filtered, washed with water, and Vacuum drying at 60°C gave the desired target 5-bromo-4-chloro-2-aminobenzoic acid (II, 28.62 g, yield 98%).
[0033]
[0034] NMR data of 5-bromo-4-chloro-2-aminobenzoic acid: 1 H-NMR (300MHz, DMSO-d 6 ): δ=7.90(s, 1H,—H-6); 7.02(s, 1H, H-3). 13 C-NMR (75MHz, DMSO-d 6 ):δ=167.69(CO 2 H); 151.23(C-2); 137.79(C-4); 13 5.37(C-6); 117.13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com