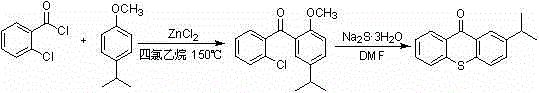
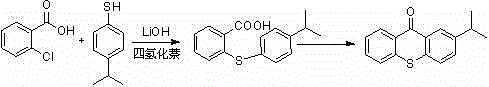
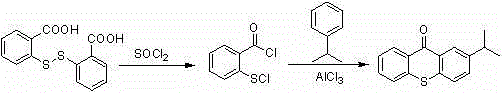The preparation method of 2-isopropylthioxanthone
A technology of isopropylthioxanthone and p-isopropylthiophenol is applied in the field of preparation of photoinitiator 2-isopropylthioxanthone, which can solve the difficulty of increasing post-processing and reduce the efficiency of photoinitiation , Large amount of concentrated sulfuric acid, waste acid and other problems, to achieve the effect of convenient post-processing, easy operation and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: Preparation method of 2-isopropylthioxanthone
[0040] Add 30.4g (0.20mol) of 4-isopropylthiophenol, 80ml of toluene, and 9.6g (0.24mol) of sodium hydroxide into a 500ml four-necked bottle, install a reflux dehydration device, stir, heat and reflux to separate water, and react for 2 hours Finally, 4-isopropylthiophenol is completely converted into sodium 4-isopropylthiophenate, the temperature is lowered to below the boiling point of the solvent, the water is separated, and the water separator is removed, and 33.0g (0.24mol) of o-chlorobenzonitrile is added, Heating to reflux reaction, TLC or liquid phase monitoring reaction, after the reaction, cool down to room temperature to obtain intermediate 2-(4-isopropylthiophenol group) benzonitrile solution, slowly add 30ml 93% sulfuric acid dropwise in a water bath, After 30 minutes of dripping, slowly heat up and heat up to 100°C for reaction, TLC or liquid phase monitoring of the reaction, after the reaction i...
Embodiment 2
[0041] Embodiment 2: Preparation of 2-isopropylthioxanthone
[0042] Add 30.4g (0.20mol) of 4-isopropylthiophenol, 80ml of toluene, and 9.6g (0.24mol) of sodium hydroxide into a 500ml four-necked bottle, install a reflux dehydration device, stir, heat and reflux to separate water, and react for 2 hours Finally, 4-isopropylthiophenol is completely converted into sodium 4-isopropylthiophenate, the temperature is lowered to below the boiling point of the solvent, the water is separated, and the water separator is removed, and 33.0g (0.24mol) of o-chlorobenzonitrile is added, Heating to reflux reaction, TLC or liquid phase monitoring reaction, after the reaction, cooled to room temperature to obtain intermediate 2-(4-isopropylthiophenol group) benzonitrile solution, slowly drop 35ml 95% sulfuric acid in a water bath, After 30 minutes of dripping, slowly heat up and heat up to 100°C for reaction, TLC or liquid phase monitoring of the reaction, after the reaction is complete, lower ...
Embodiment 3
[0043] Embodiment 3: Preparation of 2-isopropylthioxanthone
[0044] Add 30.4g (0.20mol) of 4-isopropylthiophenol, 80ml of xylene, and 9.6g (0.24mol) of sodium hydroxide into a 500ml four-necked bottle, install a reflux dehydration device, stir, heat and reflux to separate water, and react After 2 hours, 4-isopropylthiophenol was completely converted into sodium 4-isopropylthiophenate, lowered the temperature to below the boiling point of the solvent, separated the water, removed the water separator, and added 33.0g (0.24mol) o-chlorobenzonitrile , heating to reflux reaction, TLC or liquid phase monitoring reaction, after the reaction, cool down to room temperature to obtain intermediate 2-(4-isopropylthiophenol) benzonitrile solution, slowly drop 35ml 95% sulfuric acid in a water bath After 30 minutes of dripping, slowly heat up and heat up to 100°C for reaction. TLC or liquid phase monitors the reaction. After the reaction is complete, cool down and add 40ml of water, stir f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com