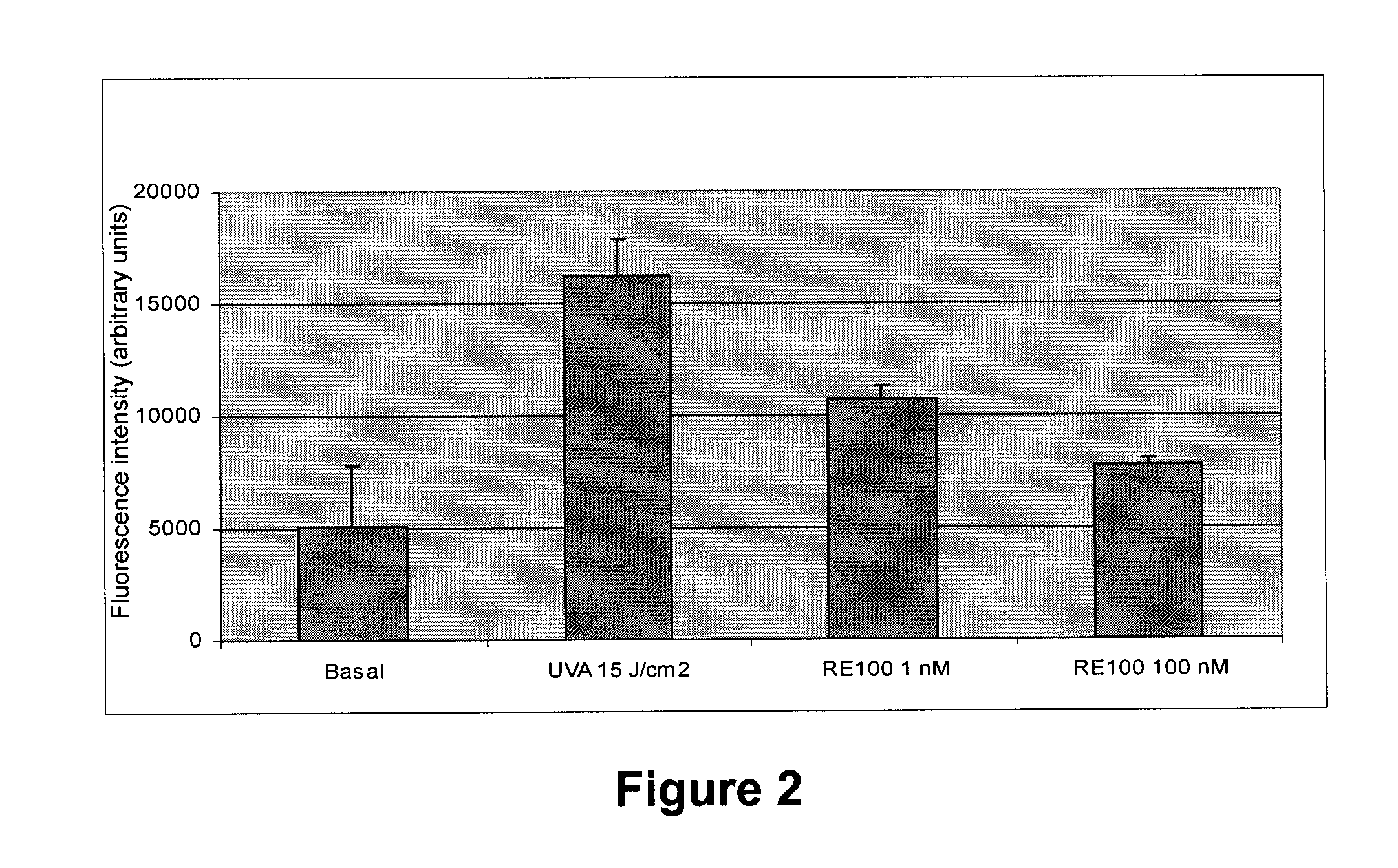
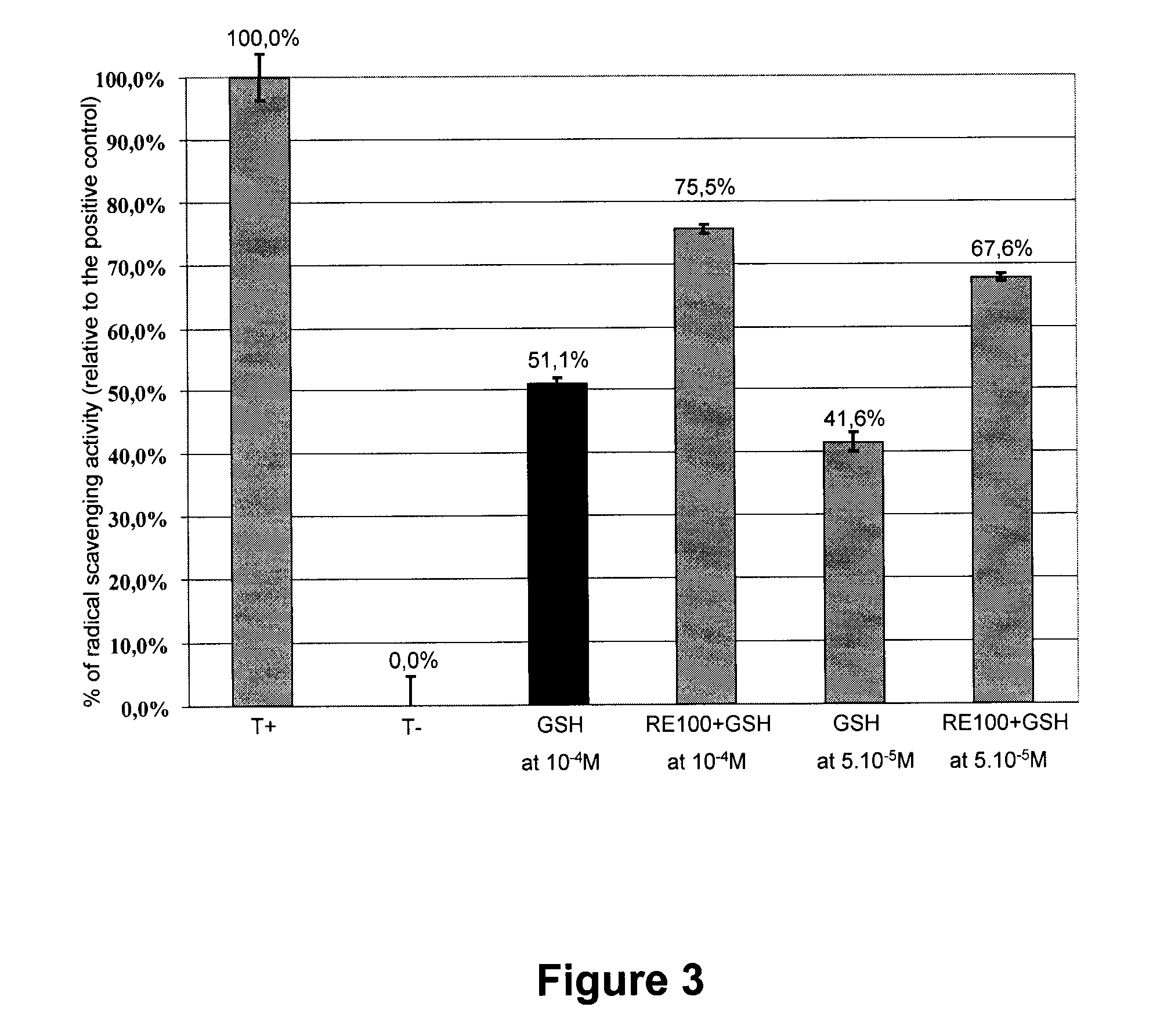
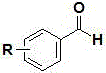
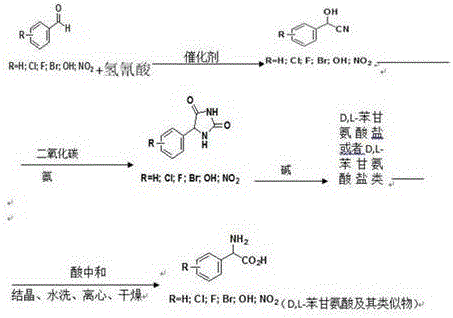
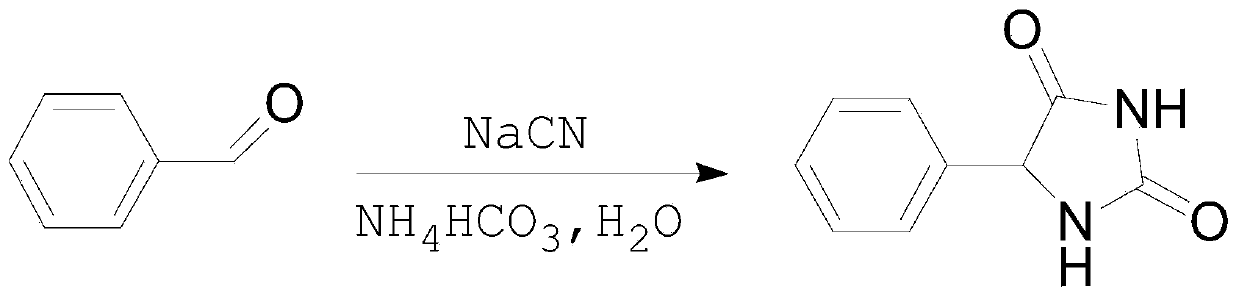
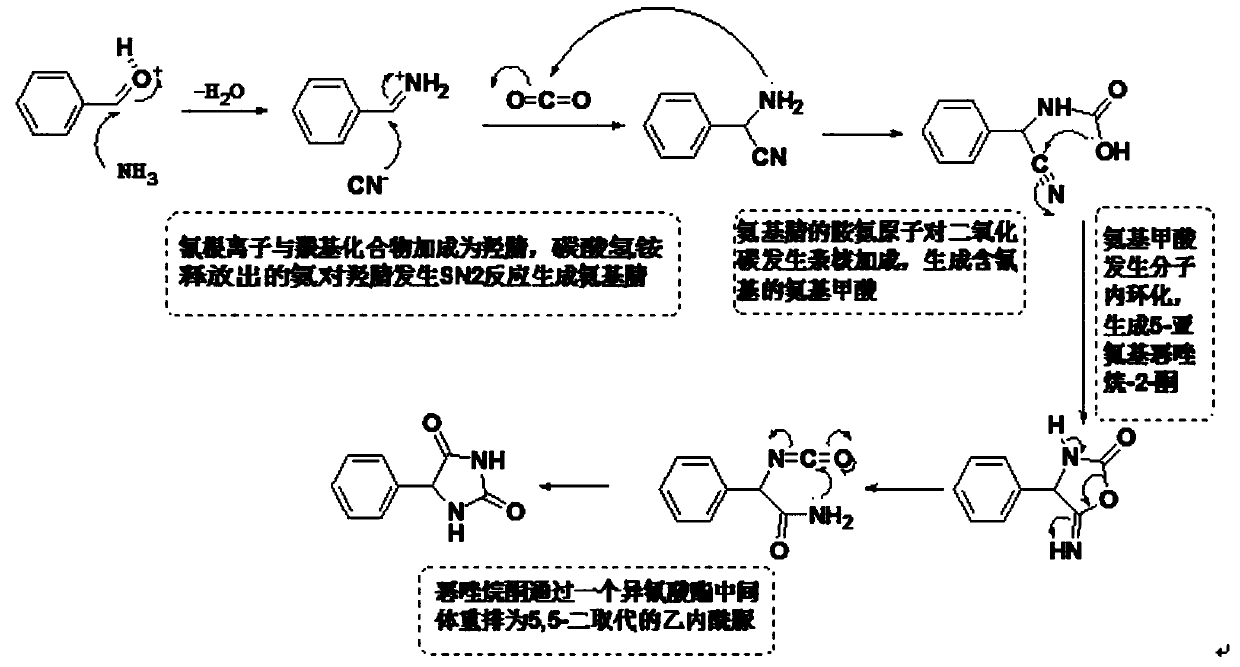
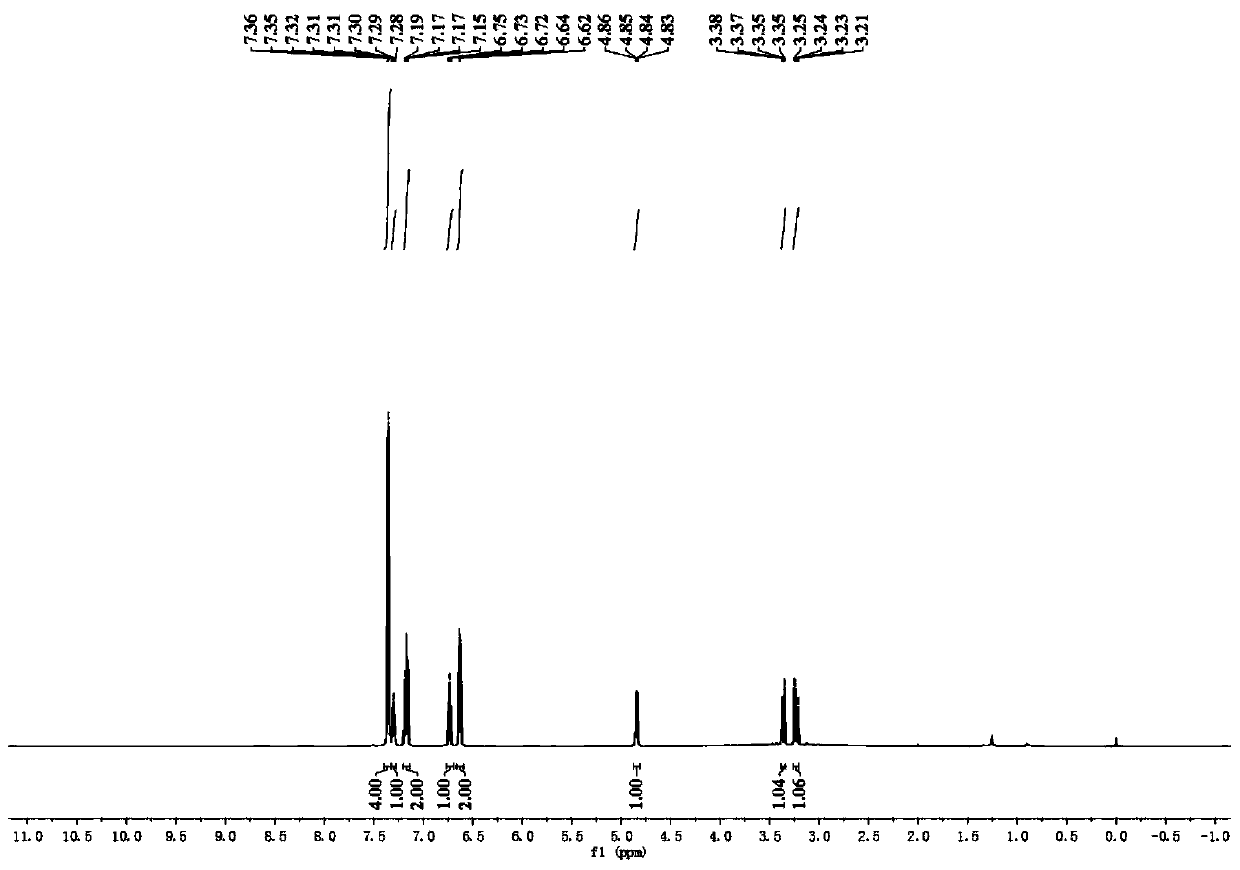
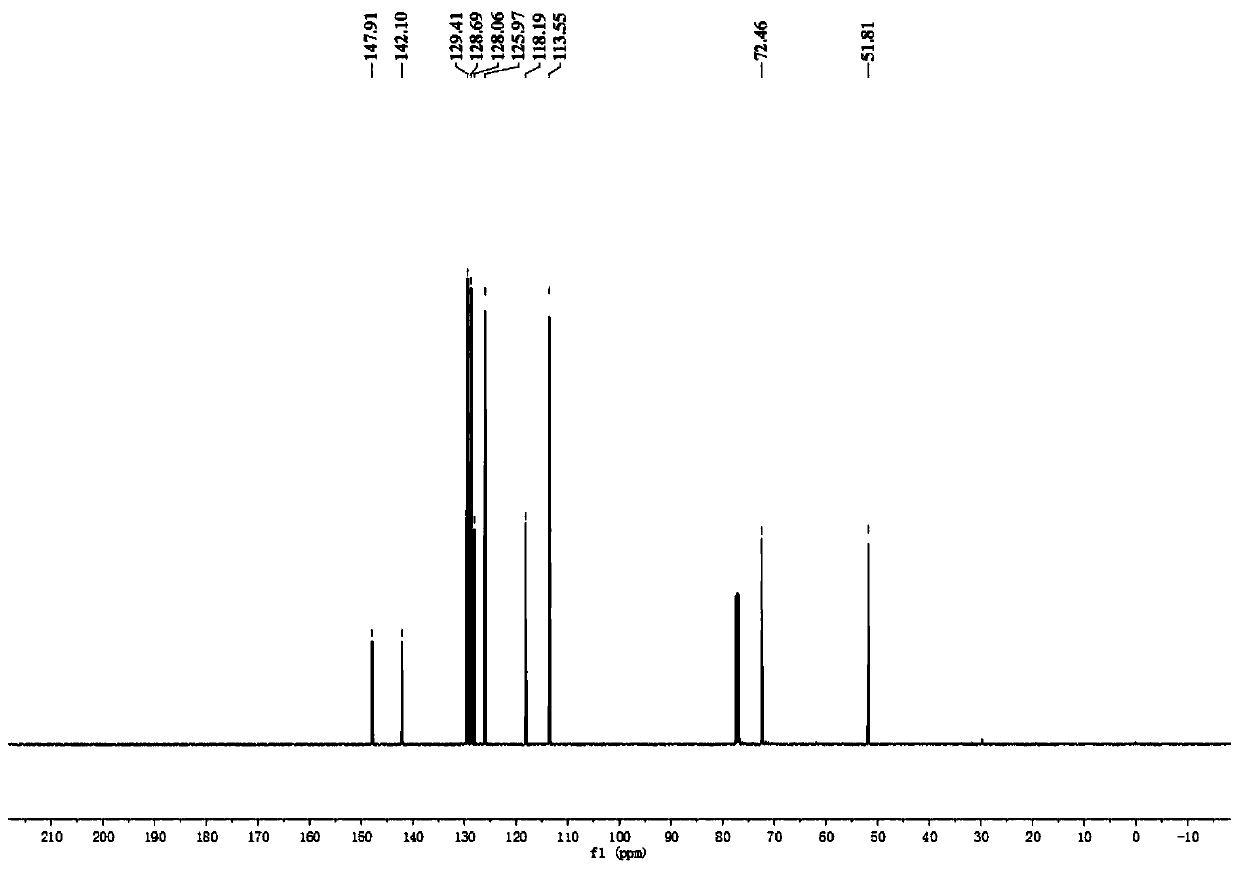
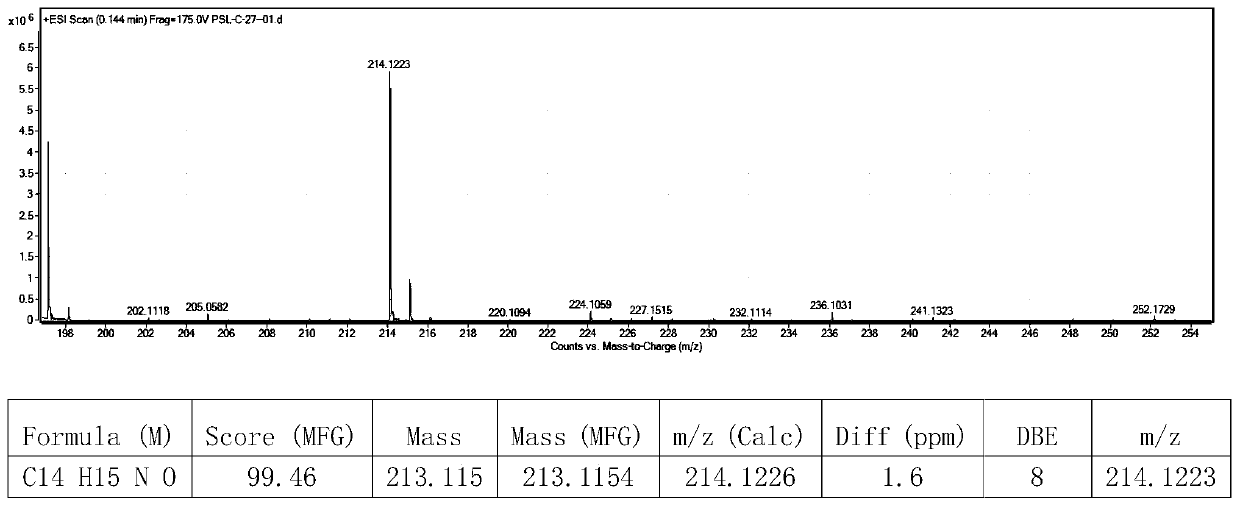
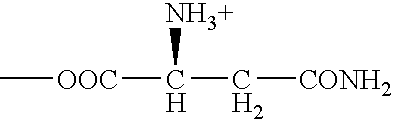
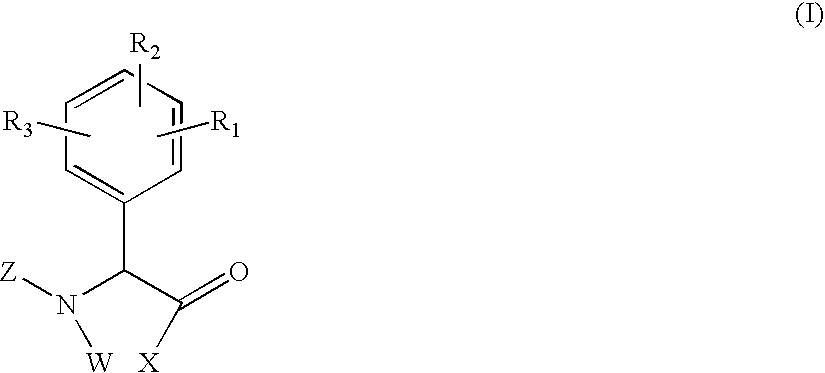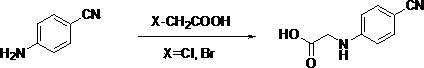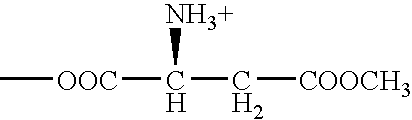Patents
Literature
95 results about "L-phenylglycine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Compositions for Regulation of Hair Growth
InactiveUS20080254055A1Reduce frequencyImprove shaving efficiencyBiocideCosmetic preparationsPersonal carePhytic acid
Personal care composition comprising at least one hair growth regulating compound selected from the group consisting of glyceryl dilaurate, apigenin, tetrahydrocurcumin, oleanolic acid, azelaic acid, sulforaphane, canavanine, pyridoxal 5-phosphate, phytic acid, tannic acid, grape seed extract, NG-nitro-L-arginine-methyl ester, benzamidine, sodium butyrate, betulinic acid, polyornithine, polyarginine, fisetin, jasmonates, methyl-jasmonate, cis-jasmone, caffeic acid phenethyl ester, delphinidin, ethyl abietate, esculetin, sorbic acid methyl ester, canaline, N-formyl-methionine, N-formyl-alanine, taurine, palmitoyl carnitine, undecanol, undecylenic acid, rutin, fusidic acid, phenyl pyruvic acid, L-isoleucine, phenyl glycine, silibinin, silymarin, L-ascorbic acid-6-palmitate, N-undecylenoyl-L-phenylalanine, and salts, derivatives and mixtures of any of the foregoing; and a dermatologically-acceptable carrier.
Owner:THE PROCTER & GAMBLE COMPANY
Compositions comprising phenyl-glycine derivatives
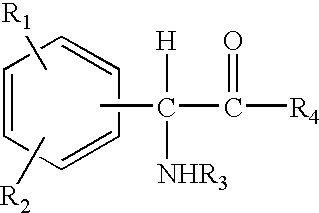
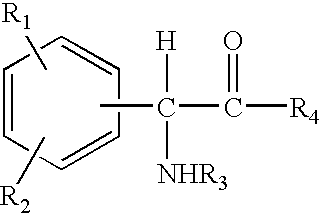
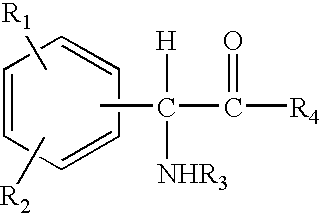
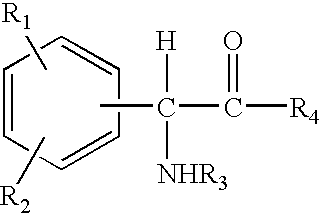
The invention relates to compositions and methods useful for treating a variety of cosmetic conditions and dermatological disorders, where the composition includes a phenyl glycine derivative represented by the following formula:wherein, R1 and R2 are independently H, I, F, Cl, Br, OH, SH, NH2, NHNH2, alkyl, aralkyl, alkoxy, acetoxy, acyloxy group having 1 to 9 carbon atoms, and being attached at the 2, 3 or 4 position of the phenyl group, whereby when R1 and / or R2 are OH, SH, NH2, they may be acetylated or acylated with 1 to 9 carbon atoms; R3 is H, formyl, acetyl, propanoyl, acyl, alkyl, aralkyl or an aryl group having 1 to 9 carbon atoms; R4 is OH, NH2, NHOH, NHNH2, or OR; where R is an alkyl, aralkyl or aryl group having 1 to 9 carbon atoms; the H attached to any carbon or nitrogen atom may be substituted by I, F, Cl, Br, OH, SH, NH2, NHNH2, an alkyl, aralkyl, alkoxy or acyl group having 1 to 9 carbon atoms. Phenyl-glycine and its derivatives may be present as isomeric D or L, non-isomeric or racemic DL, as a free acid, salt, lactone, amide or ester form.
Owner:YU RUEY J +1
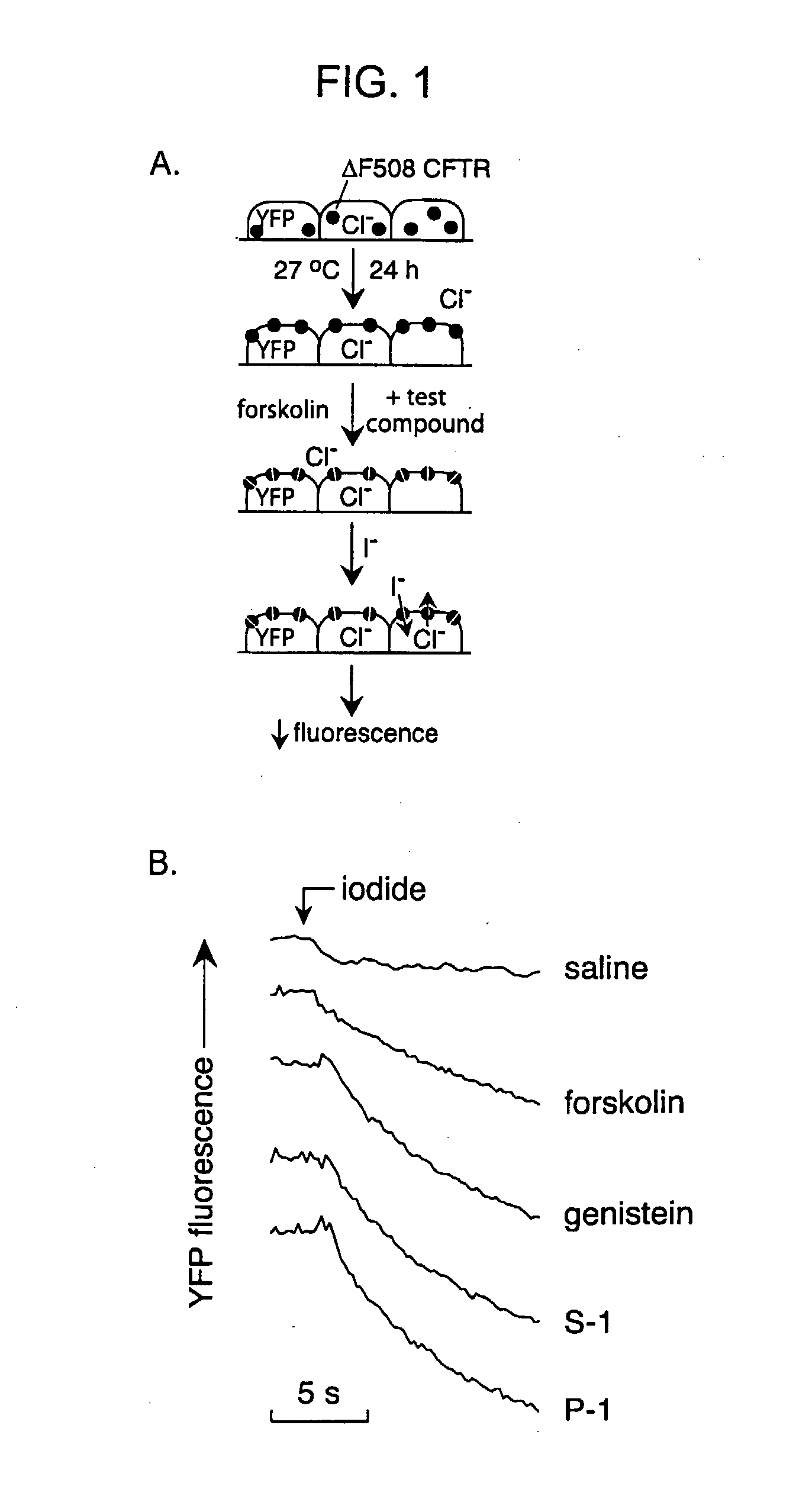
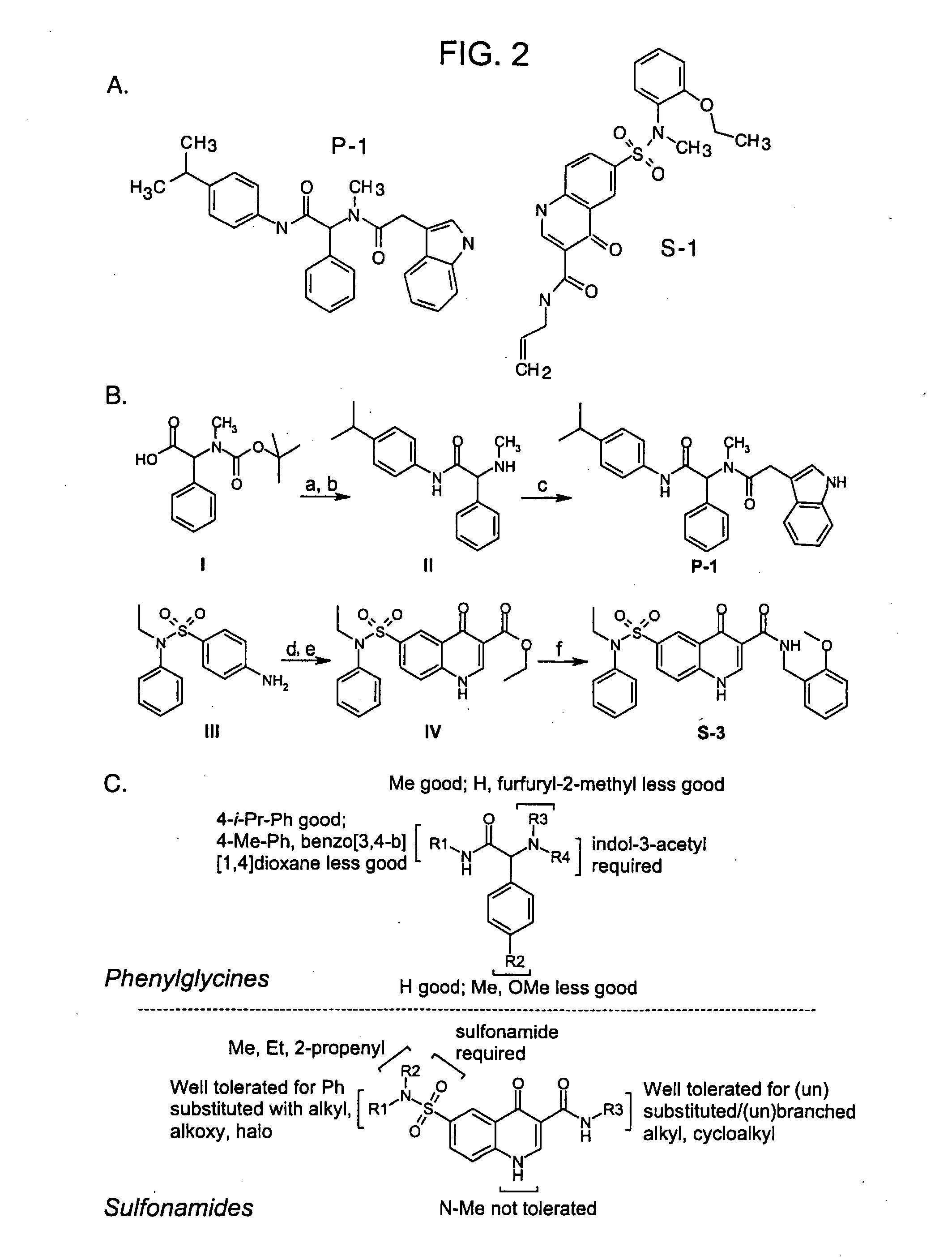
Compounds Having Activity in Increasing Ion Transport by Mutant-Cftr and Uses Thereof
ActiveUS20080319008A1High activityGood cell permeabilityBiocideOrganic chemistryCystic fibrosis lungsCystic fibrosis
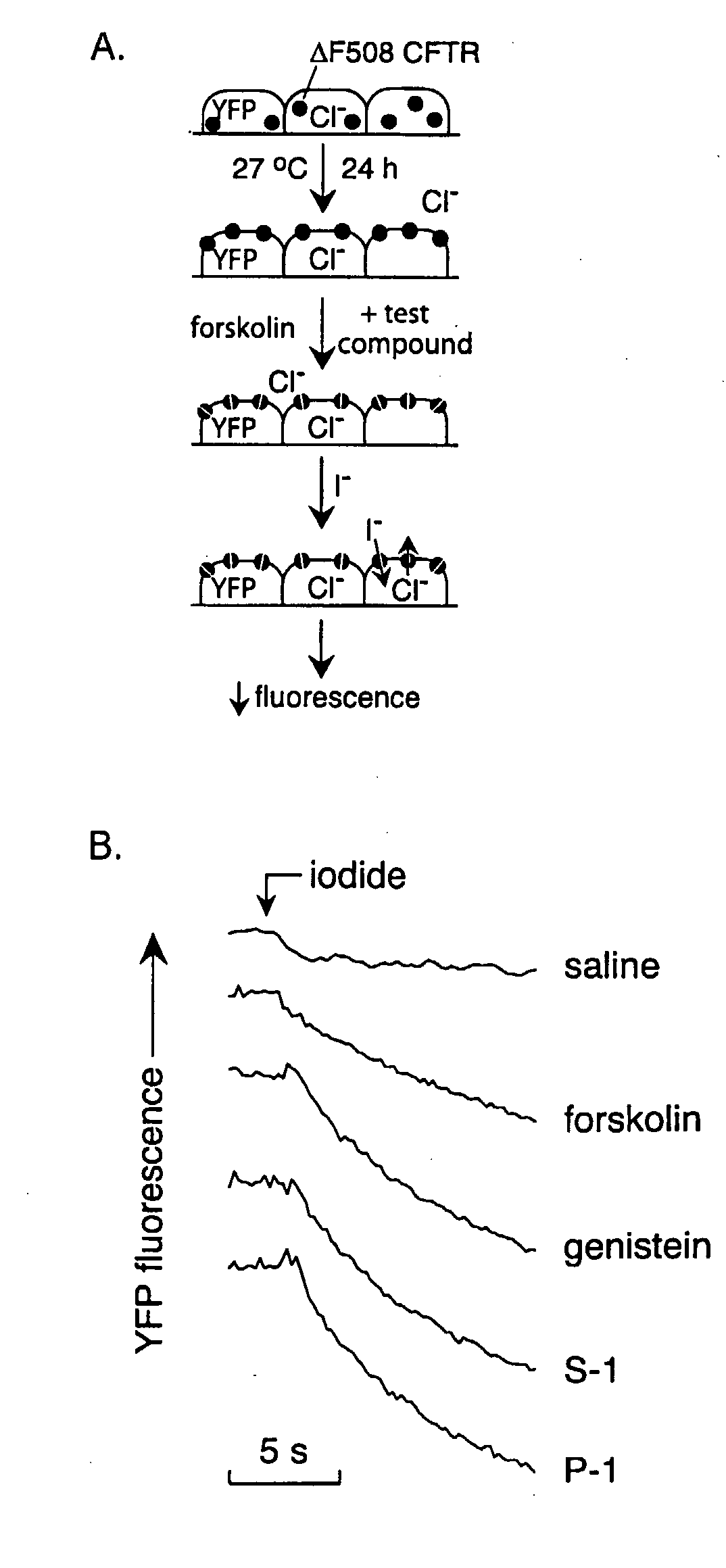
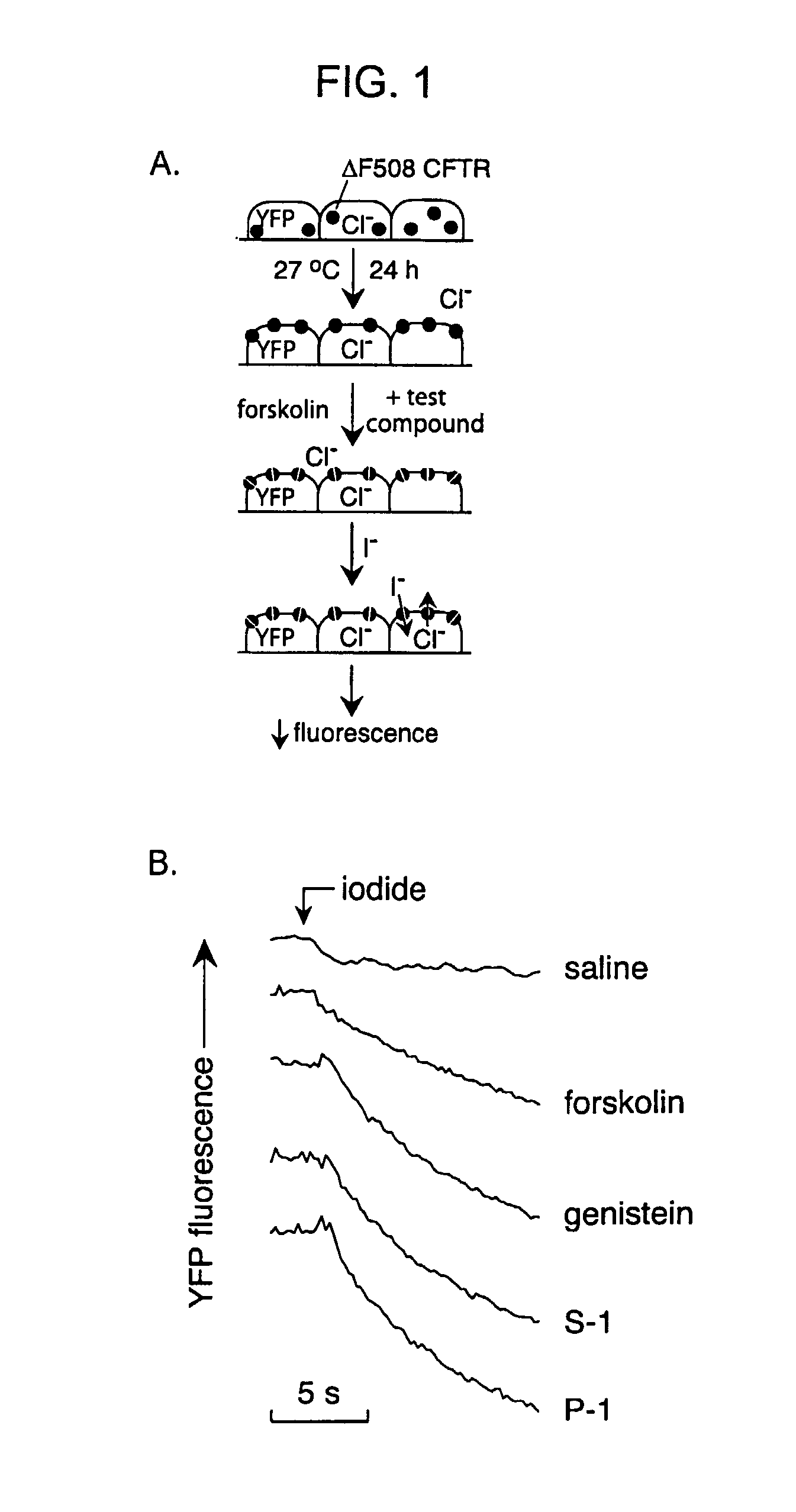
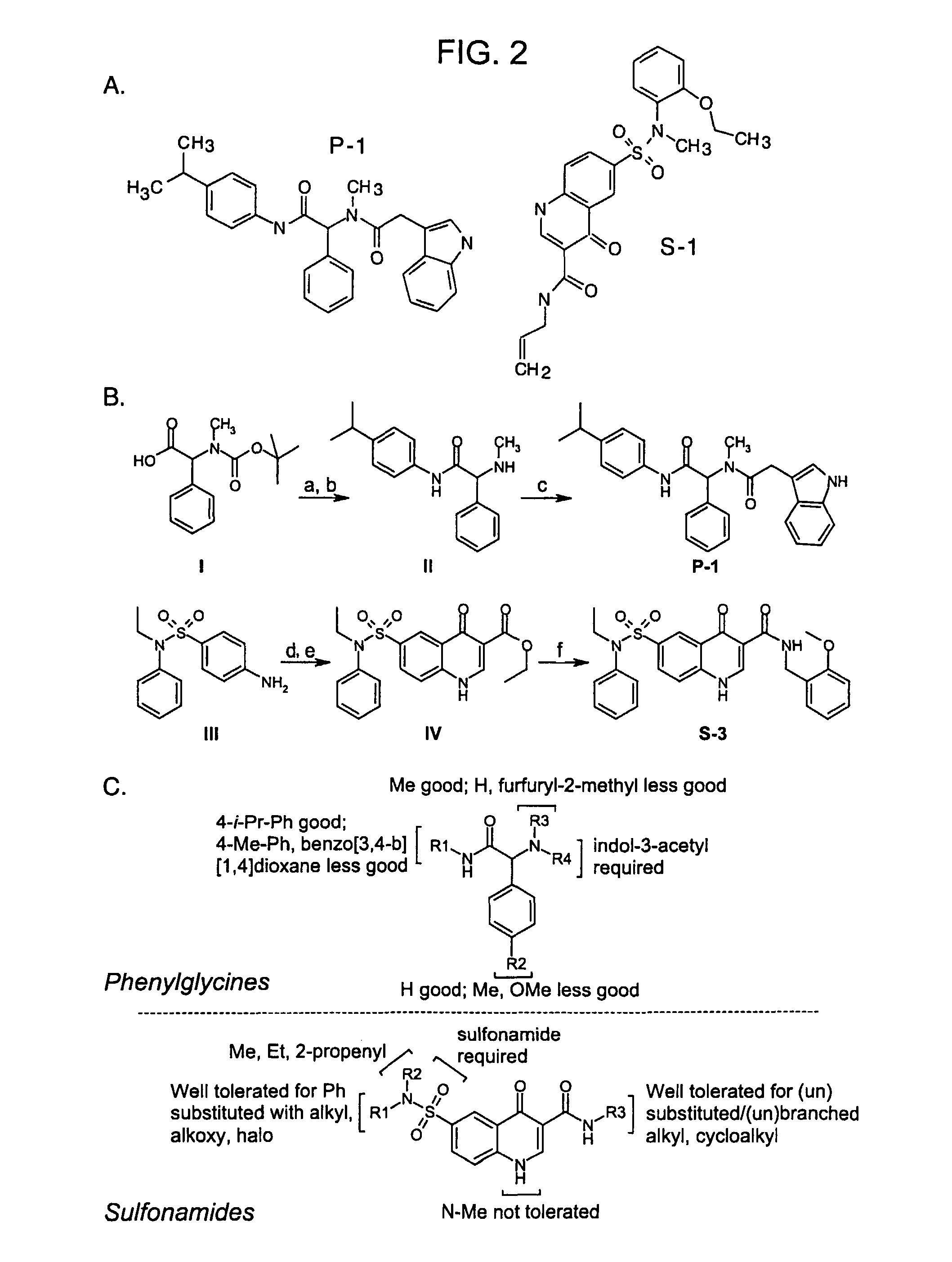
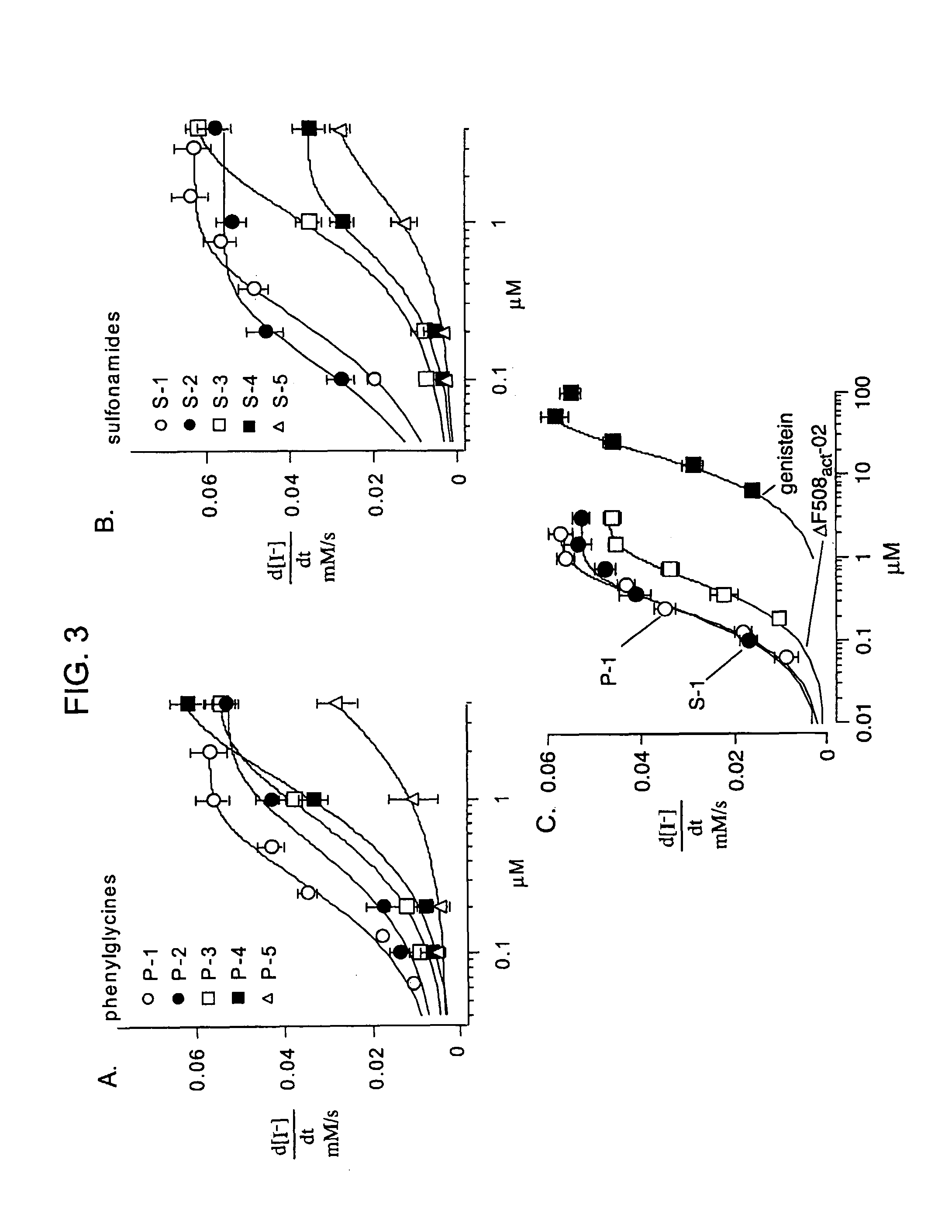
The invention provides compositions, pharmaceutical preparations and methods for increasing activity (e.g., ion transport) of the mutant cystic fibrosis transmembrane conductance regulator protein (mutant-CFTR), e.g., ΔF508 CFTR, G551D-CFTR, G1349D-CFTR, or D1152H-CFTR, that are useful for the treatment of cystic fibrosis (CF). The compositions and pharmaceutical preparations of the invention may comprise one or more phenylglycine-containing compounds or sulfonamide-containing compounds of the invention, or an analog or derivative thereof.
Owner:RGT UNIV OF CALIFORNIA
Compositions comprising phenyl-glycine derivatives
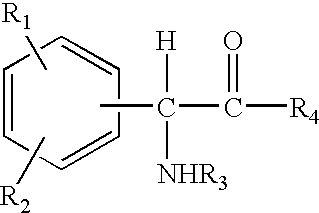
The invention relates to compositions and methods useful for treating a variety of cosmetic conditions and dermatological disorders, where the composition includes a phenyl glycine derivative represented by the following formula: wherein, R1 and R2 are independently H, I, F, Cl, Br, OH, SH, NH2, NHNH2, alkyl, aralkyl, alkoxy, acetoxy, acyloxy group having 1 to 9 carbon atoms, and being attached at the 2, 3 or 4 position of the phenyl group, whereby when R1 and / or R2 are OH, SH, NH2, they may be acetylated or acylated with 1 to 9 carbon atoms; R3 is H, formyl, acetyl, propanoyl, acyl, alkyl, aralkyl or an aryl group having 1 to 9 carbon atoms; R4 is OH, NH2, NHOH, NHNH2, or OR; where R is an alkyl, aralkyl or aryl group having 1 to 9 carbon atoms; the H attached to any carbon or nitrogen atom may be substituted by I, F, Cl, Br, OH, SH, NH2, NHNH2, an alkyl, aralkyl, alkoxy or acyl group having 1 to 9 carbon atoms. Phenyl-glycine and its derivatives may be present as isomeric D or L, non-isomeric or racemic DL, as a free acid, salt, lactone, amide or ester form.
Owner:YU RUEY J +1
Use of amino acids for treatment of various conditions
InactiveUS20060094785A1Effective treatmentImprove toleranceOrganic active ingredientsBiocidePhysiologyAlloisoleucine
A method of treating a patient for a condition characterized by symptoms that can be alleviated by interfering with or supplementing the activity of endogenous ligands on the a2S subunit of a voltage gated calcium channel, said method comprising: administering to a patient experiencing the condition an amount of one or more of L-norleucine, L-isoleucine, L-alloisoleucine, L-methionine, Lleucine, 2-cyclohexylglycine, 2-phenylglycine, 2-amino-2-norbornane carboxylic acid, 1-aminocyclohexane carboxylic acid, 2-aminoheptanoic acid, 2-aminocaprylic acid, and 2-aminononanoic acid under conditions effective to treat the condition, wherein when the condition is a hot flash or a symptom of hormonal variation, the compound is not L-leucine.
Owner:UNIVERSITY OF ROCHESTER
Penicillin G acylase immobilized with a crosslinked mixture of gelled gelatin and amino polymer
PCT No. PCT / EP96 / 03253 Sec. 371 Date Jan. 15, 1998 Sec. 102(e) Date Jan. 15, 1998 PCT Filed Jul. 16, 1996 PCT Pub. No. WO97 / 04086 PCT Pub. Date Feb. 6, 1997Penicillin G acylase is immobilized by covalent bonding to a crosslinked mixture of a gelled gelling agent such as gelatin and a polymer containing free amino groups such as alginate amine, chitosan or polyethylene imine. The immobilized penicillin G acylase provides a higher synthesis / hydrolysis ratio as compared to immobilizing with other carriers when producing beta -lactam derivatives by a condensing reaction of an amino beta -lactam with an acylating agent. The acylating agent may be a derivative of D-phenylglycine, a derivative of D-p-hydroxyphenylglycine or a derivative of D-2,5-dihydro-phenylglycine. Examples of beta -lactam derivatives that can be produced are amoxycillin, ampicillin, cephaclor, cephadroxil, cephprozil, cephalexin and cephradine.
Owner:GIST BROCADES NV
Compounds having activity in increasing ion transport by mutant-CFTR and uses thereof
ActiveUS7939558B2Good cell permeabilityImprove ion permeabilityBiocideOrganic chemistryCystic fibrosisChemistry
The invention provides compositions, pharmaceutical preparations and methods for increasing activity (e.g., ion transport) of the mutant cystic fibrosis transmembrane conductance regulator protein (mutant-CFTR), e.g., ΔF508 CFTR, G551D-CFTR, G1349D-CFTR, or D1152H-CFTR, that are useful for the treatment of cystic fibrosis (CF). The compositions and pharmaceutical preparations of the invention may comprise one or more phenylglycine-containing compounds or sulfonamide-containing compounds of the invention, or an analog or derivative thereof.
Owner:RGT UNIV OF CALIFORNIA
Photopolymerizable resin compositions and use thereof
InactiveUS6495298B1Excellent color characteristicImpression capsPhotosensitive materialsResistAdditive ingredient
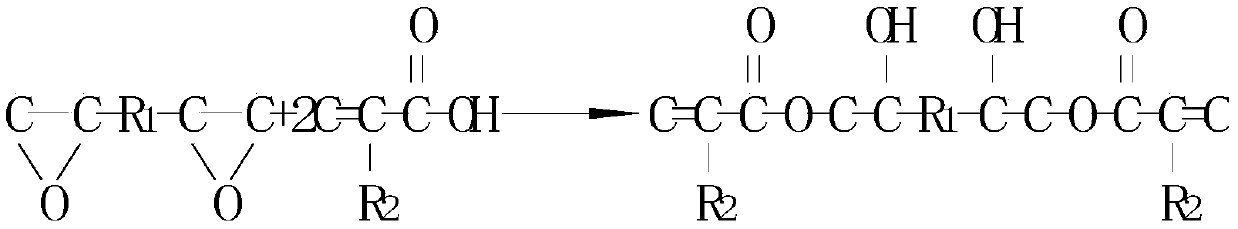
This invention relates to a photopolymerizable resin composition containing resin component (A) composed of a resin and / or a resin-forming ingredient and a photopolymerization initiator (B) wherein the component (A) comprises an addition-polymerizable compound (A1) having at least two ethylenically unsaturated groups and the photopolymerization initiator (B) comprises a diaminobenzophenone compound (B1), an N-phenylglycine compound (B2), and at least one kind of compound selected from a group of a 3,3',4,4'-tetra(alkylperoxycarbonyl)benzophenone (B3), 2-methyl-1-[4-(thiomethyl)phenyl]- 2-morpholinopropan-1-one (B4), and a 1,3,5-triazine derivative (B5) containing at least one trihalomethyl group as substituent. A photopolymerizable resin composition of this invention excels in resolution, adhesion of patterns, development latitude, and curing on the surface and inside and can be used advantageously in insulating films, colored films, inks for color filters, resists for semiconductors, and insulating spacers for touch panels.
Owner:NIPPON STEEL CHEMICAL CO LTD
Construction of leucine dehydrogenase mutants and application thereof
ActiveCN108559735AImprove hydrophobicityImprove rigidityOxidoreductasesFermentationGenetically engineeredGenetic engineering
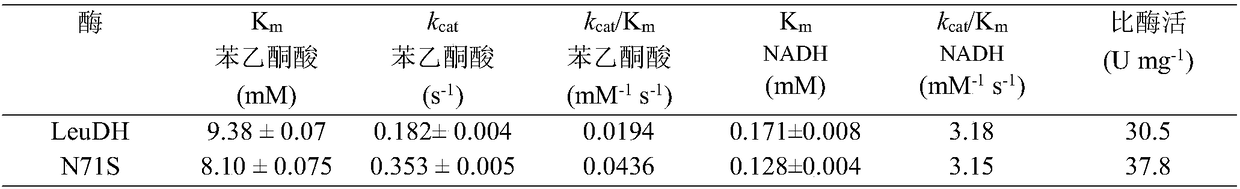
The invention provides construction of leucine dehydrogenase mutants and application thereof and belongs to the field of genetic engineering. The invention provides three leucine dehydrogenase mutantsof SEQ ID NO.4, SEQ ID NO.6 and SEQ ID NO.8, and application of the mutants and genetically engineered bacteria producing the mutants in preparation of optical pure chiral L-alpha-amino acid throughammoniation reduction of alpha-ketoacid. The invention has the advantages that the L-alpha-amino acid prepared by ammoniation reduction from the leucine dehydrogenase mutants or the genetically engineered bacteria has high catalytic activity and stability, high-optical purity L-alpha-amino acids can be synthesized (ee is greater than 99%), the conversion rate of L-phenylglycine catalyzed by mutantenzymes is improved by 2.62 times, and a practical and effective strategy is provided for industrial production.
Owner:JIANGNAN UNIV
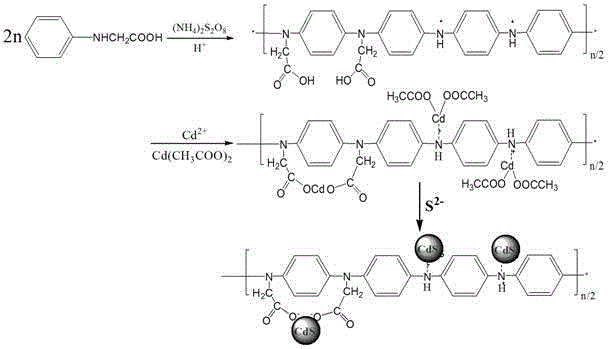
Preparation method of N-substituted carboxyl polyaniline/cadmium sulfide quantum dot composite material
InactiveCN105968347AThe force between the interface is strengthenedImprove easy reunionLuminescent compositionsOrganic dyeIn situ polymerization
The invention relates to a preparation method of an N-substituted carboxyl polyaniline grafted cadmium sulfide quantum dot composite material. The method takes N-phenyl glycine as a monomer, and comprises the steps of introducing a cadmium source into an in-situ polymerization system of the N-phenyl glycine so as to generate a cadmium-containing precursor; then introducing a sulfur source to prepare the chemically grafted N-substituted carboxyl polyaniline cadmium sulfide quantum dot composite material by utilizing a mechanism of nucleation between cadmium and sulfur. The electron transport rate of the composite material prepared by the method is much higher than that of cadmium sulfide and polyaniline, the photo-corrosion resistance of the cadmium sulfide is improved, the composite material is capable of carrying out photovoltaic conversion and treating organic dye by means of photocatalytic degradation under visible light, and the dissolving property and stability of the material are improved. The obtained composite material can be applied to the fields such as photocatalysts, sensors and solar cells.
Owner:EAST CHINA JIAOTONG UNIVERSITY
Novel peptides, use thereof in cosmetic and cosmeceutic applications, and compositions comprising same
InactiveUS20100310484A1Preventing and delaying and reducing and treating effect of agingCosmetic preparationsAntipyreticEnantiomerSide chain
A peptide of formula I (SEQ ID NO: 1): Lip-A-Gly-His-B-R (I) wherein: Lip is a lipoyl residue of R or S configuration; A is absent or is a lysine residue of configuration L or D; GIy is a glycine residue; His is a histidine residue of configuration L or D; B is a lysine residue of configuration L or D, or a lysine residue of configuration L or D in which the NH2 group of the side chain comprises a modification, wherein said modification is (i) a replacement with a hydrogen or (ii) a modification with a protecting group selected from the group consisting of acetyl, benzoyl, tosyl, sulfonyl benzene, benzyloxycarbonyle and palmitoyl; wherein R is O(Z) or N(Z′)(Z′), and wherein Z, Z′ and Z′ are independently of each other a hydrogen or a protecting group selected from the group consisting of methyl, ethyl, propyl, phenyl, hexyl, decyl and hexadecyl, or a racemate, an enantiomer or a diastereomer thereof, or a mixture thereof, or a salt thereof. A peptide of formula II (SEQ ID NO: 2): Lip-A-His-B-C-Trp-R (II) wherein: Lip is a lipoyl residue of configuration R or S; His is a histidine residue of configuration L; Trp is a tryptophane residue of configuration L; A is absent, is an amino acid residue of configuration L or D selected from the group consisting of a lysine residue, an alanine residue, a glutamic acid residue and a glycine residue, or is a spacer of formula: NH—(CH2)n-CO— wherein n is an integer comprised between 2 and 14; B is an aromatic amino acid residue of configuration D selected from the group consisting of a phenylalanine residue, a homophenylalanine residue, a tryptophane residue, a β-(1-Naphthyl)-alanine residue, a β-(2-Naphthyl)-alanine residue and a phenylglycine residue; C is a basic amino acid residue of configuration L selected from the group consisting of an arginine residue, a lysine residue, an ornithine residue and a homoarginine residue; wherein R is O(Z) or N(Z′)(Z′),
Owner:LUCAS MEYER COSMETICS CANADA
Preparation method for D, L-phenylglycine and analogue thereof
ActiveCN106380415ASave inorganic saltReduce pollutionCarboxylic acid nitrile preparationOrganic compound preparationBenzaldehydeBenzyl cyanide
The invention provides a preparation method for D, L-phenylglycine and an analogue thereof. According to the method, benzaldehyde, an analogue thereof and hydrocyanic acid are adopted as raw materials and subjected to cyanidation reaction, and then 2-hydroxy-benzyl cyanide or 2-hydroxy-benzyl cyanide analogue (cyanohydrin for short) is generated. Cyanohydrin reacts with carbon dioxide and the aqueous solution of ammonia, and then 5-phenyl-hydantoin and an analogue thereof (hydantoin for short) are generated. hydantoin is successively subjected to steam stripping, alkaline hydrolysis, steam stripping, decolorization, neutralization, crystallization, washing, centrifuging, drying and the like to obtain D, L-phenylglycine and the analogue thereof. Compared with the prior art, the preparation method for D, L-phenylglycine and the analogue thereof can significantly and effectively reduce the pollution, and fewer inorganic salt by-products are generated. Meanwhile, the prepared D, L-phenylglycine and the analogue thereof are high in product yield and high in purity. Counted in benzaldehyde and the analogue thereof, the yield of D, L-phenylglycine and the analogue thereof is larger than or equal to 96%, and the product purity is larger than or equal to 99%. Meanwhile, the process flow is simple and feasible, so that the method is worthy of market popularization and application.
Owner:NINGXIA UNISPLENDOUR TIANHUA METHIONINE CO LTD
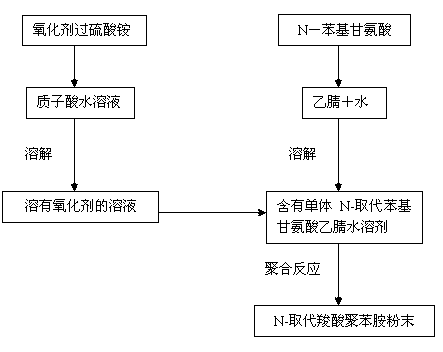
Chemical oxidation preparation method for novel N-substituted carboxyl polyaniline
The invention discloses a chemical oxidation preparation method for novel N-substituted carboxyl polyaniline. According to the method, the N-substituted carboxyl polyaniline polymer is prepared through a chemical oxidation method by taking N-phenylglycine as a monomer, and the method comprises the following steps: dissolving a certain amount of N-phenylglycine (N-AN) monomer in a mixed solution of acetonitrile and water in a certain volume; dissolving a certain amount of oxidant ammonium persulfate (NH4)3S2O3 in a prepared hydrochloric acid aqueous solution in a certain size; and slowly dripping the solution dissolved with the oxidant ammonium persulfate into a monomer, so that the monomer is completely reacted, and treating to obtain the N-substituted carboxyl polyaniline powder. The polymer prepared by the method has the yield of 89 weight percent and the electric conductivity of 1.5*10<-1>S / cm, and the N-substituted carboxyl polyaniline has excellent dissolving performance. The obtained polymer powder can be applied to conductive high polymer materials in the fields of secondary batteries, sensors, solar cells and the like.
Owner:EAST CHINA JIAOTONG UNIVERSITY
Phenylglycine derivatives useful as serine protease inhibitors
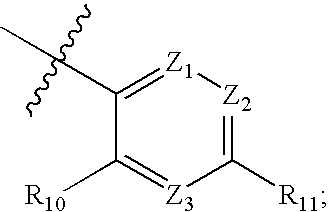
Compounds having the formula (I),or a stereoisomer or pharmaceutically-acceptable salt, or hydrate thereof, are useful as factor VIIa inhibitors, wherein X is —NR6S(O)pR16; W is hydrogen or —(CR7R8)q—W1; W1 is hydrogen or a bond with R6; Z is a 5-membered heteroaryl group, a five to six membered heterocyclo or cycloalkyl group, a 9 to 10 membered bicyclic aryl or heteroaryl, or a six membered aryl or heteroaryl, and R1, R2, R3, R6, R7, and R16 are as defined in the specification.
Owner:BRISTOL MYERS SQUIBB CO
Racemization of optically active 2-substituted phenyl glycine esters
InactiveUS20040073057A1Organic compound preparationAmino-carboxyl compound preparationGlycineCombinatorial chemistry
A process for preparing racemic mixtures containing nearly equal amounts of stereo isomers of compounds of formula (I), or their salts, by heating an enantiomerically enriched material with thionyl chloride. A required useful enantiomer may thereby be recovered from unwanted mother liquors that would otherwise be otherwise be discarded.
Owner:USV LTD
Method for preparing modified polyaniline grafted functionalized graphene composite cadmium sulfide quantum dots
InactiveCN106750278AGood dispersionAccelerated corrosionOrganic-compounds/hydrides/coordination-complexes catalystsIce waterUltrasonic dispersion
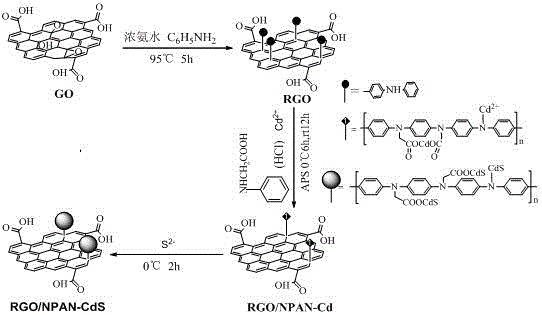
The invention relates to a method for preparing modified polyaniline grafted functionalized graphene composite cadmium sulfide quantum dots. The method comprises the steps of preparing graphene oxide (GO), carrying out ultrasonic dispersion to obtain a uniform solution, putting a certain volume of stronger ammonia water and aniline into the GO solution, carrying out a reaction at a certain temperature so as to obtain functionalized reduced graphene oxide (RGO), uniformly mixing a certain amount of RGO, N-phenylglycine and a cadmium source in an in-situ polymerization system with a weakly acidic atmosphere, adding a certain amount of ammonium persulfate into the mixture as an oxidant, carrying out a reaction so as to prepare a cadmium-containing composite material precursor, and finally, introducing a sulfur source under ice water bath conditions, thereby preparing an N-substituted polyaniline carboxylate grafted graphene cadmium sulfide quantum dot photoelectric material by using a mechanism of nucleation between sulfur and cadmium. According to the composite photoelectric material prepared by the method provided by the invention, the agglomeration of cadmium sulfide and graphene is inhibited through covalent grafting, the light corrosion resistance of cadmium sulfide is improved, the transfer speed of electrons is accelerated, and the efficiency of photoelectric conversion under visible light is increased.
Owner:EAST CHINA JIAOTONG UNIVERSITY
Single cell factory capable of efficiently synthesizing L-phenylglycine as well as construction and application of single cell factory
ActiveCN108103038AOptimize regeneration rateReduce the cost of trainingMicroorganism based processesOxidoreductasesEscherichia coliCell factory
The invention discloses a single cell factory capable of efficiently synthesizing L-phenylglycine as well as construction and application of the single cell factory and belongs to the technical fieldof microorganisms. Firstly, efficient expression of leucine dehydrogenase obtained from Bacillus cereus in escherichia coli is realized, and site-directed mutation is carried out to obtain a mutant N71S with a remarkably improved reduction property; a mutant enzyme and a formate dehydrogenase mutant are co-expressed in the escherichia coli to form an intracellular in-situ co-factor NADH (Nicotinamide Adenine Dinucleotide) circulating system; the expression amount of the formate dehydrogenase mutant is optimized and controlled through a promoter and an RBS (Ribosomal Binding Site) sequence to successfully construct a recombinant escherichia coli single cell factory; the single cell factory is subjected to whole-cell conversion to prepare the L-phenylglycine. The method disclosed by the invention has the advantages of simple and rapid conversion process, low cost, no byproduct and easiness for separation and purification; when conversion is carried out in a 5L fermentation tank for 4h, the yield of the L-phenylglycine can reach 105.7g / l, the conversion rate is 93.3 percent and the space-time yield of the L-phenylglycine is 26.3g / L; an actually practical and effective strategy is provided for industrial production of the L-phenylglycine.
Owner:JIANGNAN UNIV
Method for preparing green light sensitive holographic photoluminescent polymer and holographic recording dry plate
The invention is a green light sensitive holographic photopolymer and a method of preparing holographic record dry plate, where the photopolymer is composed of film-forming polyvinyl alcohol, monomeric acylamide and diacylamide, photosensitive agent phycoerythrin B, initiator N-phenylglycine and plasticizing agent. The preparing course of the holographic dry plate: firstly dissolving the polyvinyl alcohol in the distilled water to make 7% (wt) polyvinyl alcohol water solution; secondly, adding acylamide crystals and methylene diacylamide crystals as well as N-phenylglycine and plasticizing agent to the polyvinyl alcohol water solution; thirdly, adding in phycoerythrin B dissolved in the distilled water; fourthly, pouring the above sol onto a basal plate and after the water is completely volatilized, making the photopolymer holographic drying plate. It is sensitive to the green light and the environmental humidity has a little effect on it, and it has strong shrink resistance, short drying time, diffraction efficiency able to reach 65%, and exposure strength 50 mJ / sq cm and it is especially applied to dry-processing of optical holographic record.
Owner:SHANGHAI INST OF OPTICS & FINE MECHANICS CHINESE ACAD OF SCI
Preparation method of ezetimibe internmediate ketone
InactiveCN104744390AReduce manufacturing costExperimental operation safetyOrganic chemistryImproved methodPhenyl group
The invention belongs to the field of medicinal chemistry and relates to improvement of a preparation method of an important ezetimibe internmediate (4S)-3-[5-(4-fluorophenyl)-1,5-dioxo amyl]-4-phenyl-2- oxazolidinone I. According to the improved method, L-phenylglycine, p-fluorobenzoylbutanoic acid are used as raw materials and are subjected to three steps of reaction to obtain the target intermediate (4S)-3-[5-(4-fluorophenyl)-1,5-dioxo amyl]-4-phenyl-2- oxazolidinone I. The preparation method disclosed by the invention is simple to operate, low in consumption, low in raw material cost and simple and easy in post-treatment and therefore is suitable for industrial production.
Owner:NANJING NORMAL UNIVERSITY
Polyaniline-base injectable photo-thermal hydrogel
InactiveCN105457026AEasy to prepareMild reaction conditionsOrganic active ingredientsEnergy modified materialsPolymer scienceFluorescence
The invention discloses polyaniline-base injectable photo-thermal hydrogel which is binary-system hydrogel composed of alpha-cyclodextrin and a water-soluble high-polymer photo-thermal material. The water-soluble high-polymer photo-thermal material is polyethylene glycol grafted poly-N-phenylglycine comprising a main chain and a side chain, and the main chain is poly-N-phenylglycine (PPG) while the side chain is polyethylene glycol (PEG). The hydrogel has good photothermal conversion capability and can be used for photothermal therapy, meanwhile, the hydrogel can load chemotherapy drugs, photodynamic reagents and fluorescence imaging reagents to respectively obtain a photo-thermal hydrogel carry system which has chemotherapy, photodynamic therapy and imaging functions and is a biocompatible multifunctional photo-thermal response-type carrier hydrogel system, and the hydrogel has broad application prospect in drug carriers, photocontrol drug release, photothermal therapy, photodynamic therapy, tissue engineering and the like.
Owner:GUANGXI NORMAL UNIV
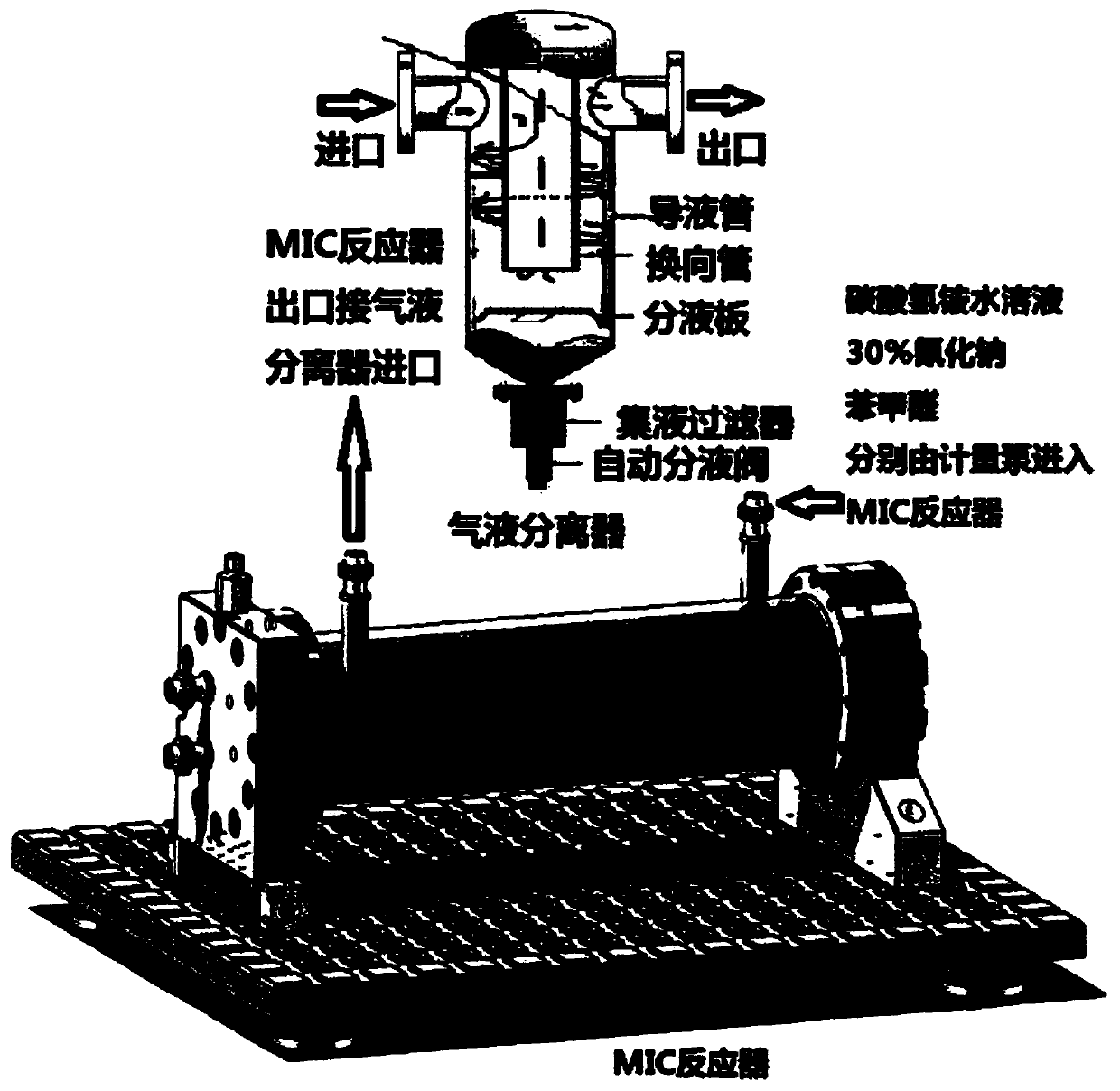
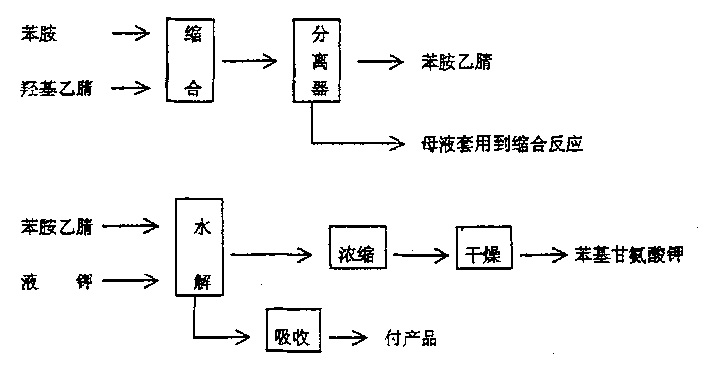
Novel method for L-phenylglycine precursor phenylhydantoin by MIC reactor
The invention relates to a novel method for an L-phenylglycine precursor phenylhydantoin by an MIC reactor. An existing process carries out intermittent production by using a reaction kettle, is morein tissue-free exhaust gas, has the probability that materials on a sealing face in a material transfer process leak and is relatively high in potential safety hazard and environmental protection risks. Ammonium hydrogen carbonate (converted in purity), sodium cyanide (30% aqueous solution) and benzaldehyde are pumped into the MIC reactor preheated to 60 DEG C in a mass ratio of 8.2: 15.8: 10 fora mixed reaction, and a prepared liquid is hydrolyzed to obtain a dl-phenylglycine. The discharge port of the MIC reactor is connected to a gas-liquid separating tank, the gas outlet of the gas-liquidseparating tank is connected to a pressure gauge to display the system pressure, and the gas-liquid separating tank is also connected to a back pressure value to back-press the whole system. By replacing solid ammonium hydrogen carbonate with an ammonium hydrogen carbonate aqueous solution, dust and tissue-free exhaust gas generated in inputting the solid ammonium hydrogen carbonate are avoided successfully. In addition, the dosage of ammonium hydrogen carbonate is reduced from 1.5 equivalent weight to 1.1 equivalent weight, so that the economical benefit is obvious.
Owner:ZHEJIANG YUNTAO BIOTECH
Green synthesis method of amino alcohol compounds under visible light catalysis
ActiveCN110550992AThe synthetic route is simpleSimple and fast operationOrganic compound preparationOrganic free radical generationSynthesis methodsOrganic synthesis
The invention discloses a green synthesis method of amino alcohol compounds under visible light catalysis, and belongs to the technical field of organic synthesis. The green synthesis method specifically comprises the following steps that S1, 90.4mg of N-phenylglycine, 1.9mg of a photocatalyst, 2ml of water and 20.4ul of benzaldehyde are added in sequence into 10ml of a dried Schlenk tube; S2, three times of air extraction operation is conducted on a reaction bottle to ensure that no water or oxygen exists in a reaction tube before sealing; S3, the reaction bottle is placed under Blue LED irradiation, mixing is conducted until TLC detecting reaction is finished; and S4, 5 ml of ethyl acetate is correspondingly extracted for 4 times, organic phases are collected, anhydrous Na2SO4 is used for drying, appropriate silica gel is added and concentrated under decompression, and obtained residues are purified through column chromatography to obtain the product amino alcohol compounds. Comparedwith the prior art, the green synthesis method has the characteristics of simple synthesis route, easy and convenient operation, little environmental pollution, mild reaction conditions, good controllability of reaction conditions, and wide application range of substrates.
Owner:HANGZHOU NORMAL UNIVERSITY
Efficient co-production strategy of L-phenylglycine and gluconic acid
ActiveCN106119272AGood market demandThe conversion process is fast and efficientOxidoreductasesFermentationEscherichia coliGluconic acid
The invention provides a method for co-producing L-phenylglycine and gluconate through single expression and co-expression of glucose dehydrogenase and L-leucine dehydrogenase in escherichia coli through utilizing a recombinant escherichia coli enzyme method and a whole cell method. The method is as follows: a glucose dehydrogenase gene and an L-leucine dehydrogenase gene are used for constructing recombinant single expression and co-expression carriers and are transformed into a gene engineering bacterium, namely the escherichia coli. The circulation of cofactors in a transformation system can be promoted through utilizing a recombinant bacterium enzyme method and the whole cell method; only a few of exogenous cofactors are added or the exogenous cofactors do not need to be used, and the L-phenylglycine and gluconic acid, which have high additional value, are co-produced by substrates including benzoylformic acid and glucose through utilizing a cofactor cyclic regeneration system; a transformation process is simple and rapid and low in cost. When transformation is carried out in a 5L fermentation tank for 2h to 4h, the yields of the L-phenylglycine and the gluconic acid, obtained by the method, can respectively reach 58.8g / L and 75.6g / L, and an actual and effective strategy is provided for industrial production.
Owner:JIANGNAN UNIV
Aqueous solution developed dry film photoresist
InactiveCN110941141AReduce usageHigh strengthPhotosensitive materials for photomechanical apparatusPolymer scienceAdhesive
The invention relates to the technical field of circuit board production and manufacturing. The aqueous solution developed dry film photoresist is prepared from the following components: an acrylate adhesive A, epoxy acrylate B1, urethane acrylate B2, a photocuring monomer B3, a radical initiator C, an active monomer D, N-phenylglycine, a plasticizer, an ultraviolet absorber and a thermal polymerization inhibitor, wherein the epoxy acrylate B1 accounts for 1-15% of the total mass of the dry film photoresist, and the content of carbon-carbon double bonds in the epoxy acrylate B1 is 1-30 mol; wherein the urethane acrylate B2 accounts for 1-15% of the total mass of the dry film photoresist, and the content of carbon-carbon double bonds in the urethane acrylate B2 is 1-30 mol; the hole sealingcapability of the aqueous solution developed dry film photoresist is obviously higher than that of a commercially available dry film photoresist, so that the aqueous solution developed dry film photoresist has a wide application prospect and a high market value.
Owner:ZHUHAI DYNAMIC TECH OPTICAL IND
Preparation method of N-substituted phenyl glycine
ActiveCN103992241ASimple unit operationLow equipment requirementsCarboxylic acid nitrile preparationOrganic compound preparationGlyoxylic acidGlycine
The invention discloses a preparation method of novel N-substituted phenyl glycine (dabigatran ester intermediate). The preparation method of the novel N-substituted phenyl glycine comprises the following processes: carrying out condensation on glyoxylic acid and cheap and available substituted phenylamine (1) which is taken as a starting raw material to obtain imide; meanwhile, carrying out hydrogenation reduction to obtain N-(substituted phenyl) glycine. The preparation method of the novel N-substituted phenyl glycine has the advantages that the synthetic route is not reported before, raw materials are cheap and easily available; unit operation is easy, and equipment requirement is low, so that the preparation method of the novel N-substituted phenyl glycine is applicable to industrial production.
Owner:ABA CHEM CORP
Method for synthesizing 1-acetyl-halo-indolyl-3-acetate
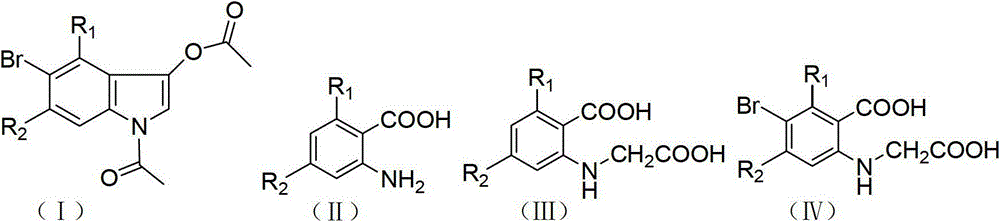
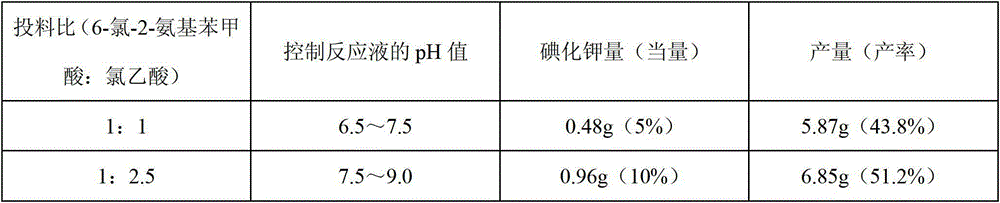
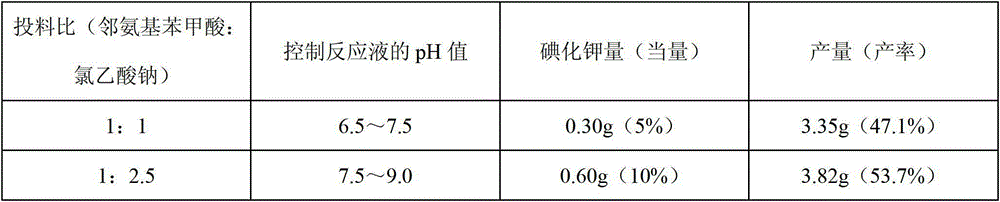
InactiveCN103145605AHigh yieldThe reaction is easy to operateOrganic chemistryStrong acidsP-Toluenesulfonic acid
The invention discloses a method for synthesizing 1-acetyl-halo-indolyl-3-acetate. According to the method, ortho-aminobenzoic acid with a general formula (II) and a chloride of ortho-aminobenzoic acid which serve as starting raw materials react with ClCH2COOH, NaOH, Na2CO3 and a catalyst KI (or NaI or the like) to produce N-(2-carboxyl)phenylglycine with a general formula (III) and a chloride of N-(2-carboxyl)phenylglycine, N-(2-carboxyl)phenylglycine bromine of a general formula (IV) and a chloride of N-(2-carboxyl)phenylglycine bromine are produced in a manner that N-(2-carboxyl)phenylglycine and the chloride of N-(2-carboxyl)phenylglycine react with N-bromosuccinimide (NBS), CH3OH, H2O and a catalyst, namely ammonium nitrate (or strong acids and weak-base salts, such as ammonium chloride, ammonium sulfate, hydrochloric acid, p-toluenesulfonic acid (p-TSA) and sulfuric acid, or protonic acid), and N-(2-carboxyl)phenylglycine bromine and the chloride of N-(2-carboxyl)phenylglycine bromine react with Ac2O and anhydrous AcNa to produce 1-acetyl-halo-indolyl-3-acetate. The method has the advantages of being safer, more environment-friendly, more time-saving, more convenient in operation, and the like.
Owner:GUANGDONG INST OF MICROORGANISM +1
Use of amino acid transporter atbo,+ as a delivery system for drugs and prodrugs
InactiveUS20040142317A1Easy to transportGood effectAntibacterial agentsOrganic active ingredientsDiseaseMedicine
The present invention has revealed the compounds transportable by ATB<0+>. Based on the information about these compounds, drugs transportable by ATB<0,+> may be designed, produced and screened. Such drugs may serve to treat and / or prevent the diseases in which NOS, phenylglycine, carnitine, D-amino NOS, phenylglycine, carnivolved. The ATB<0,+> gene may be administered to patients to be used for gene therapy of the diseases as described above.
Owner:CHUGAI PHARMA CO LTD +1
Method for producing indigo
InactiveCN1465625ASolving Wastewater ProblemsReduce energy consumptionIndigoid dyesPotassiumWastewater
The production method of indigo incldues the following steps: placing aniline and hydroxy-acetonitrile into a condensation reactor according to the mole ratio of 1:0.9-1:1.2, reacting for 2-8 hr. at 60-105 deg.C; placing all the reactants into separator to make stratification by standing still; placing lower layer oily material into crystallizing tank to make solidification, and when the aniline acetonitrile content is up to above 90%, directly adding the above-mentioned material into the hydrolysis reactor, placing the supernatnat fluid in the separator into the condensation reactor; placingthe liquid potassium and aniline acetonitrile into the reactor according to the mole ratio of 1:0.9-1:1.2, under the condition of boiling and refluxing making hydrolysis reaction, using acid adsorb the ammonia gas discharged by reaction.
Owner:上海蓝建染化有限公司 +1
Antibiotic oligopeptide mimetics
InactiveUS20180340008A1Improved oligopeptide uptakeStrong specificityAntibacterial agentsBiocideTyrosineTryptophan
Disclosed are amino acid mimetics that possess antibiotic properties in prokaryotic cells. These mimetics are coupled to one or more optionally substituted amino acids provided that at least one of the amino acids is an optionally substituted amino acid selected from the group consisting of phenylglycine, tryptophan, phenylalanine, histidine, and tyrosine.
Owner:BIOXINESS PHARMA INC
Composite gold nanoclusters phototherapy agent capable of being simultaneously used for near infrared biological windows I and II and preparation method thereof
ActiveCN109893650AImprove light-to-heat conversion efficiencyGood light and heat stabilityEnergy modified materialsPharmaceutical non-active ingredientsSinglet oxygenTherapeutic effect
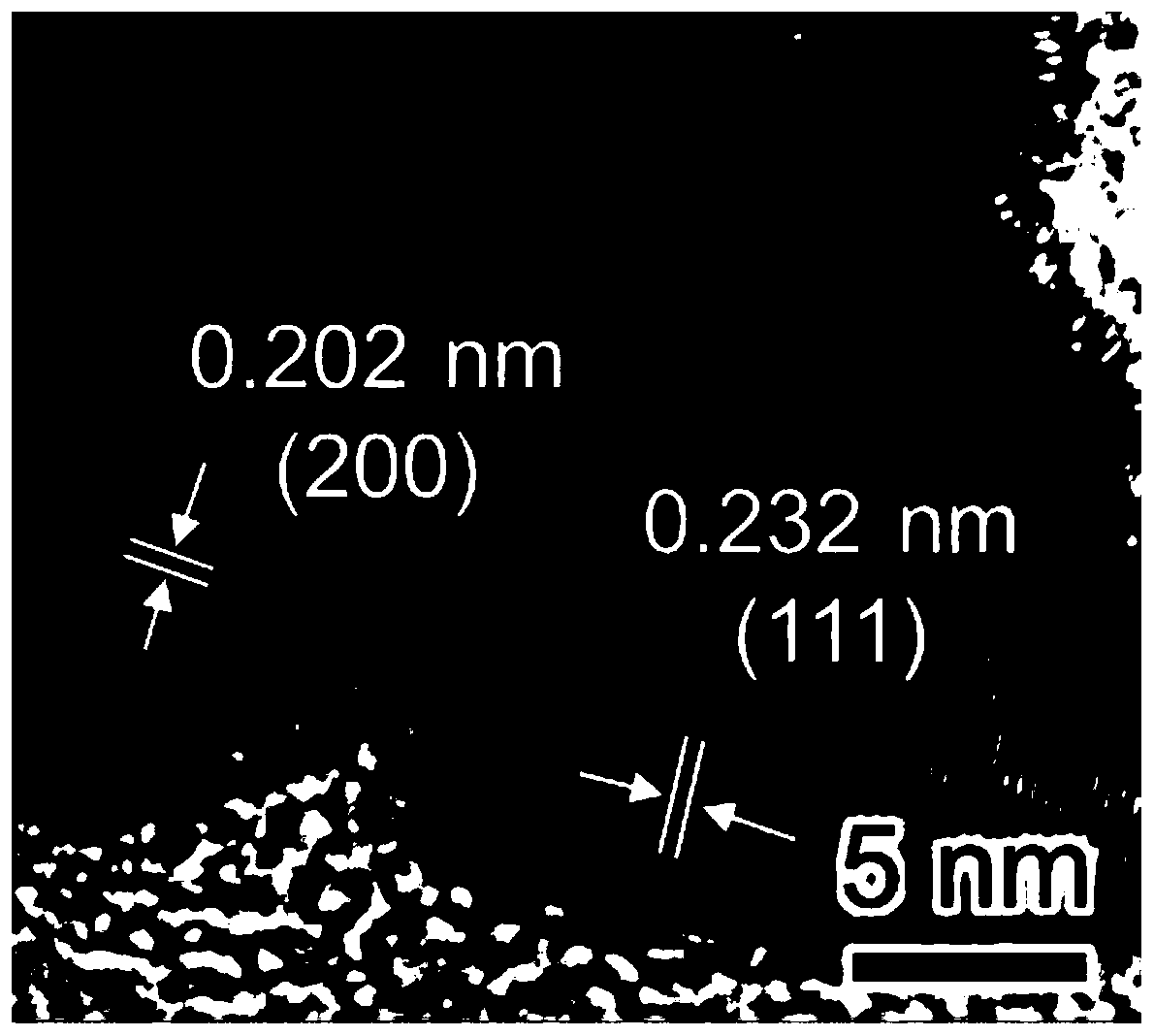
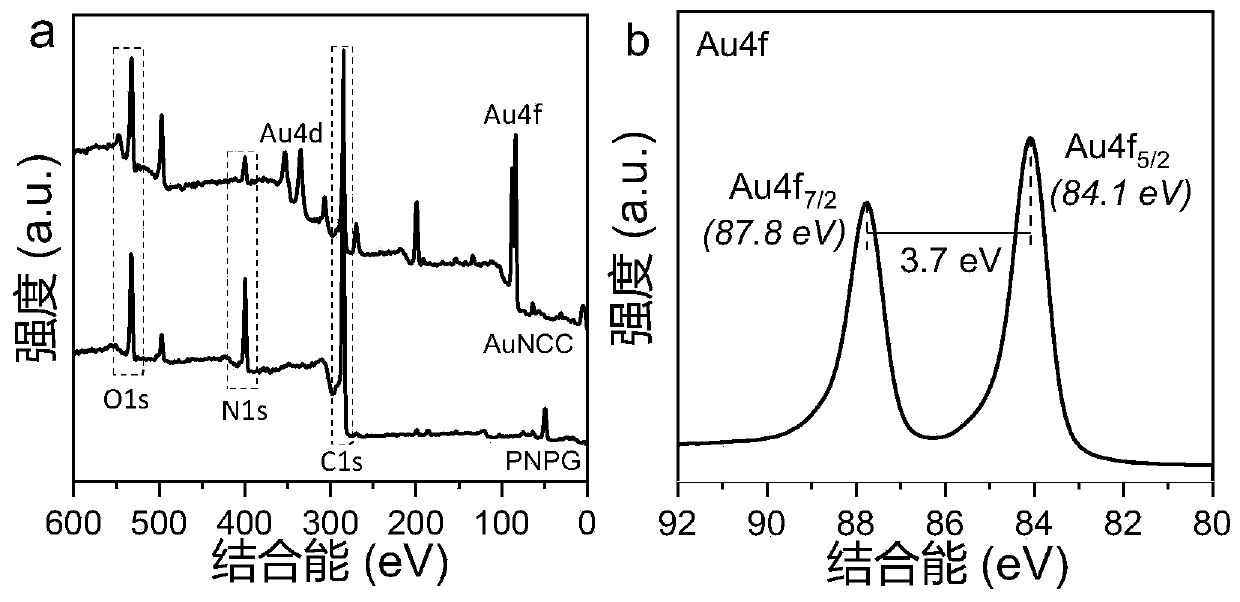
The invention provides a preparation method of a composite gold nanocluster phototherapy agent, the method comprises the following steps of: mixing aqueous solution of HAuC l4 and alkaline solution ofN-phenylglycine to obtain a gold nanocluster coated with poly N-phenylglycine. The invention provides a convenient and simple method for obtaining a composite gold nanoclusters which has effective absorption in both NIR-I and NIR-II windows at room temperature by a pot method, and the composite gold nanoclusters has the excellent photothermal conversion efficiency and photothermal stability in two near infrared rays, meanwhile, surrounding O 2 molecules can be excited by near infrared laser irradiation to generate singlet oxygen, so that the composite gold nanoclusters in the near infrared Iand II windows both have dual therapeutic effects of photothermal and photodynamic. The preparation method and the phototherapy agent are expected to provide an efficient phototherapeutic agent for cancer treatment in the future.
Owner:UNIV OF SCI & TECH OF CHINA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com