Chemical oxidation preparation method for novel N-substituted carboxyl polyaniline
A technology of chemical oxidation and polyaniline, which is applied in the field of chemical oxidation preparation of N-substituted carboxylic acid polyaniline, can solve the problems that the conductivity of polyaniline is greatly affected, the conductivity of polyaniline drops greatly, and it is not conducive to the application of polyaniline. Good Yield and Universal Applicability, Improved Solubility, Effect of High Conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
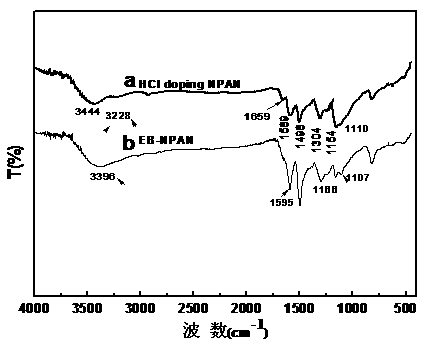
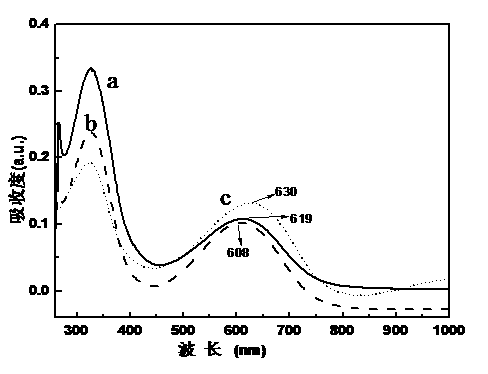
Image
Examples
Embodiment 1
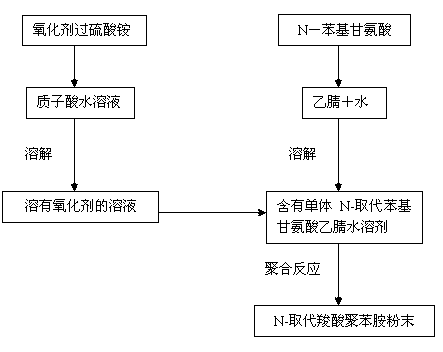
[0033] This embodiment will illustrate that the N-substituted carboxylic acid polyaniline of the present invention is in the inorganic protic acid aqueous solution, using ammonium persulfate as the oxidizing agent, the molar ratio of the oxidizing agent and the monomer is 1:1, and is realized according to the chemical oxidation polymerization reaction path .
[0034]Dissolve 4.535g (0.03mol) of N-substituted naphthylglycine monomer in 100mL of acetonitrile / water (1:1) mixed solution, and sonicate for 3 to 5 minutes to promote its dissolution. Dissolve 6.84g (0.03mol) of oxidant ammonium persulfate in 50mL of HCl solution with a concentration of 6.0mol / L as the oxidant solution. The acetonitrile aqueous solution in which the monomer was dissolved was kept at a temperature of 20°C, and after stirring for about 20 minutes, the oxidant was slowly added dropwise. After the dropwise addition was completed, the reaction was continued for 24 hours, the reaction was stopped, and the c...
Embodiment 2
[0038] This embodiment will illustrate that N-substituted carboxylic acid polyaniline of the present invention is in acetonitrile / water (1:1) hydrochloric acid solution, adopts ammonium persulfate as oxidizing agent, but the molar ratio of oxidizing agent / monomer is 0.5:1, according to It is realized by chemical oxidation polymerization reaction pathway.
[0039] Dissolve 4.535g (0.03mol) of N-substituted naphthylglycine monomer in 100mL of acetonitrile / water (1:1) mixed solution, and sonicate for 3 to 5 minutes to promote its dissolution. Dissolve 3.42 g (0.015 mol) of oxidant ammonium persulfate in 50 mL of HCl solution with a concentration of 6.0 mol / L as the oxidant solution. The acetonitrile solution in which the monomer was dissolved was kept at a temperature of 20°C, and after stirring for about 20 minutes, the oxidizing agent was slowly added dropwise. After the dropwise addition was completed, the reaction was continued for 24 hours, the reaction was stopped, and the...
Embodiment 3
[0043] Repeat Example 1, but change the protic acid medium to HNO 3 , The yield of the obtained polymer was 76.3wt%. The conductivity is 1.49×10 -1 S / cm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com