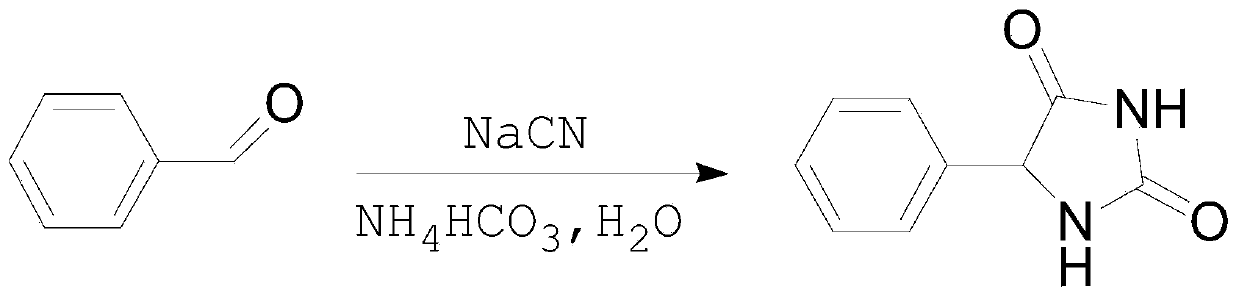
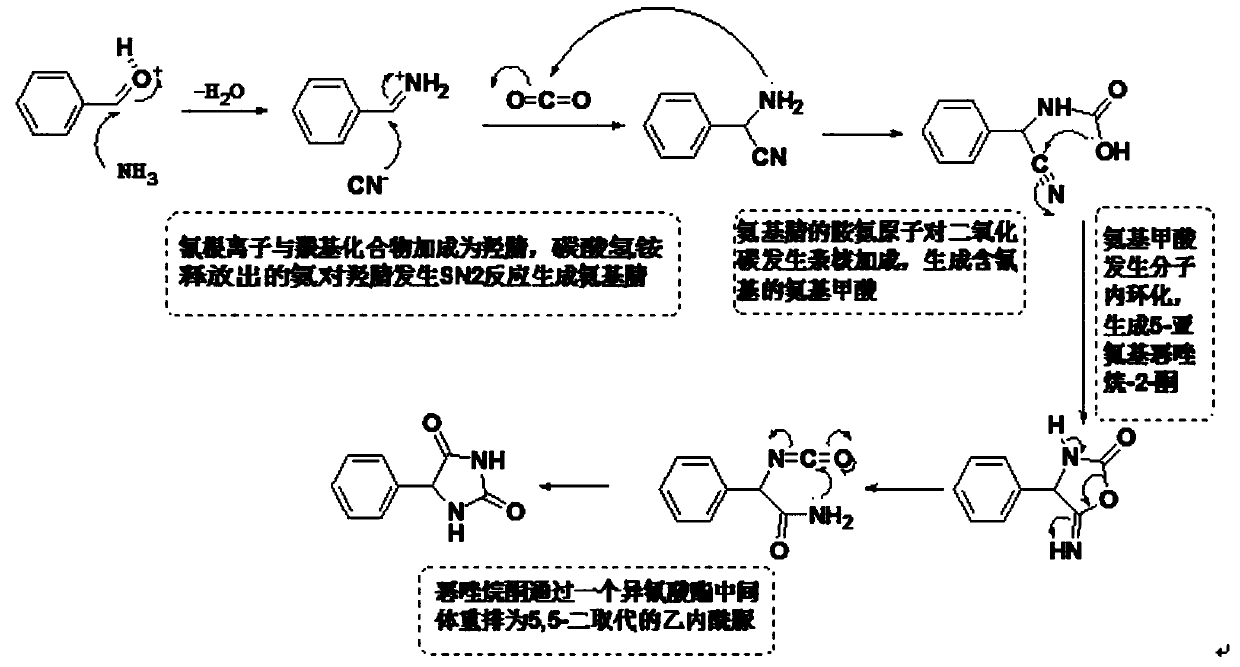
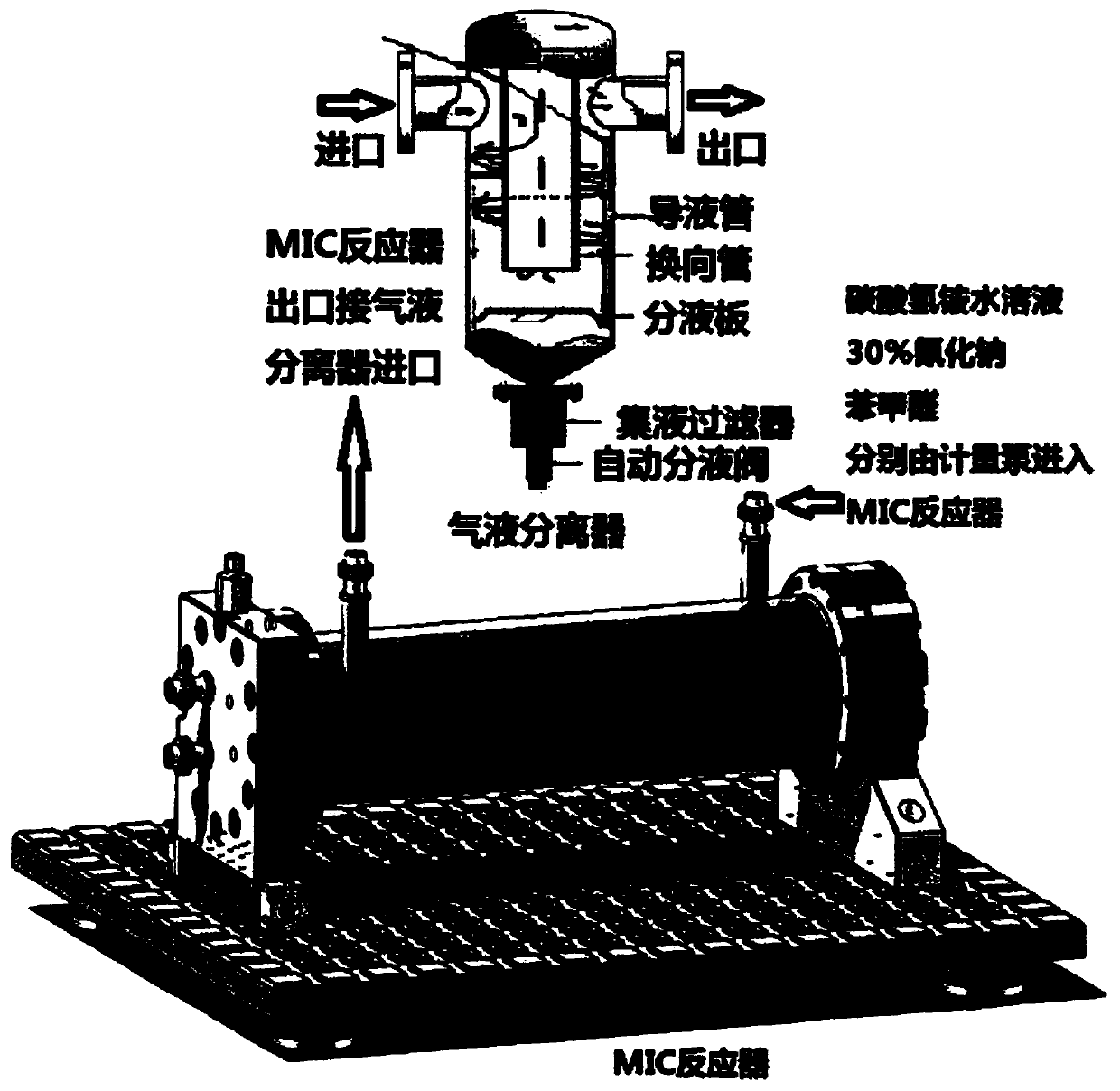
Novel method for L-phenylglycine precursor phenylhydantoin by MIC reactor
A technology of L-phenylglycine and reactor, which is applied in organic chemistry and other fields, can solve problems such as hidden safety hazards and high environmental risks, excessive unorganized waste gas, leakage of sealing surface materials, etc., and achieve slow amplification effect, significant economic benefits, and economic benefits obvious effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: 820kg ammonium bicarbonate (converting pure), 1580kg sodium cyanide (30% aqueous solution), 1000kg benzaldehyde are pumped into the MIC reactor that has been preheated to 60 ℃ by metering pump respectively, note The ammonium bicarbonate aqueous solution is first pumped into about 500kg, and then the benzaldehyde, the ammonium bicarbonate aqueous solution and 30% sodium cyanide are pumped in at the same time. The flow rate setting is based on the simultaneous pumping of the three raw materials. The preparation solution was hydrolyzed to obtain 1644 kg of derbiphenylglycine, and the test results were: loss on drying: 19.23%; sulfate radical: 2 ppm; alkali absorbance value: 0.277AU.
Embodiment 2
[0019] Ammonium bicarbonate aqueous solution, 30% sodium cyanide, and benzaldehyde are continuously pumped into the MIC reactor preheated to 60°C at the flow rate of 2800L / h, 940L / h, and 600L / h respectively, and the preparation that meets the requirements can be obtained continuously Liquid, the prepared liquid can be hydrolyzed to obtain qualified DL-phenylglycine. Note that during the continuous preparation process, always pay attention to whether the reaction solution turns blue. If it turns blue, it means that there is too much sodium cyanide in the reaction system, and it is necessary to reduce the feeding amount of 30% sodium cyanide in time.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com