Preparation method of 7-piperazinyl benzothiophene or salt thereof
A technology based on benzene and piperazine, applied in the field of preparation of 7-piperazinylbenzothiophene or its salt, can solve the problems of high production cost, expensive, low reaction yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056]
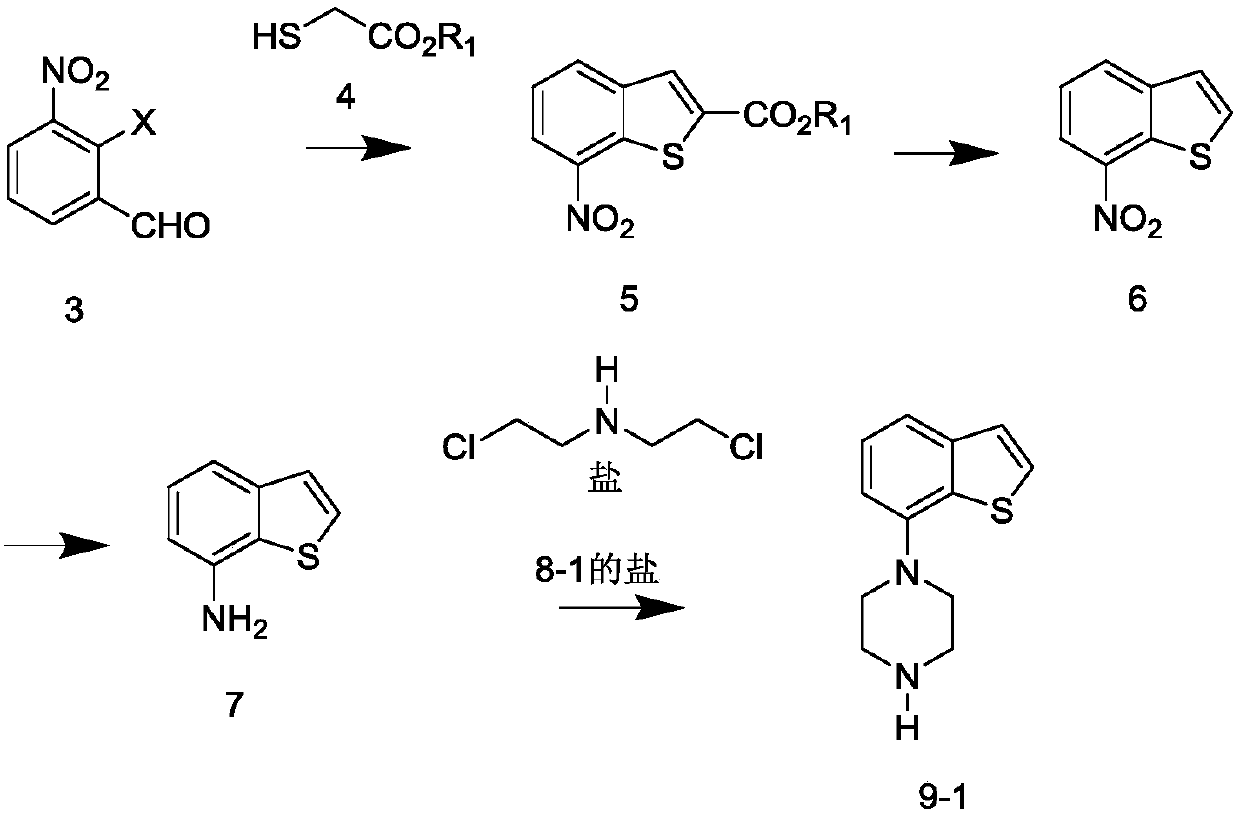
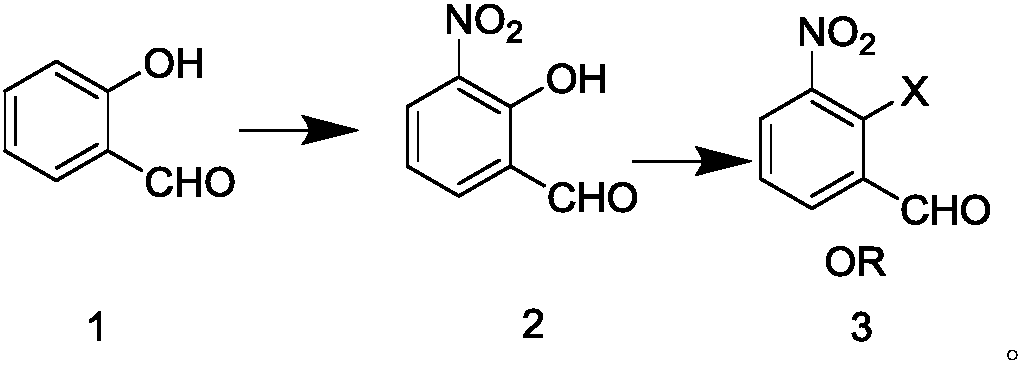
[0057] According to the literature method of Synthetic Communications; 2011, 41(20), 2985-2992, under the action of nitrate and trichloroisocyanuric acid, salicylaldehyde was selectively nitrated to obtain 3-nitrosalicylaldehyde (compound 2).
[0058] In a 100ml one-necked flask, 3-nitrosalicylaldehyde (1.67g, 0.01mol), triethylamine (4ml, 0.028mol, 2.8eq), and dichloromethane (40ml) were stirred and dissolved. After cooling in an ice bath to below 10°C, p-toluenesulfonyl chloride (1.90g, 0.01mol, 1eq) was added. After the addition was complete, the temperature was raised naturally, and the reaction was stirred for 4h. Sampling and detection, after the reaction of 3-nitrosalicylaldehyde is complete, pour the reaction solution into ice dilute hydrochloric acid, stir and separate the liquid, extract the water phase with dichloromethane, combine the organic phase, and use saturated sodium bicarbonate aqueous solution, saturated salt Washed with water, dried over anhyd...
Embodiment 2
[0060]
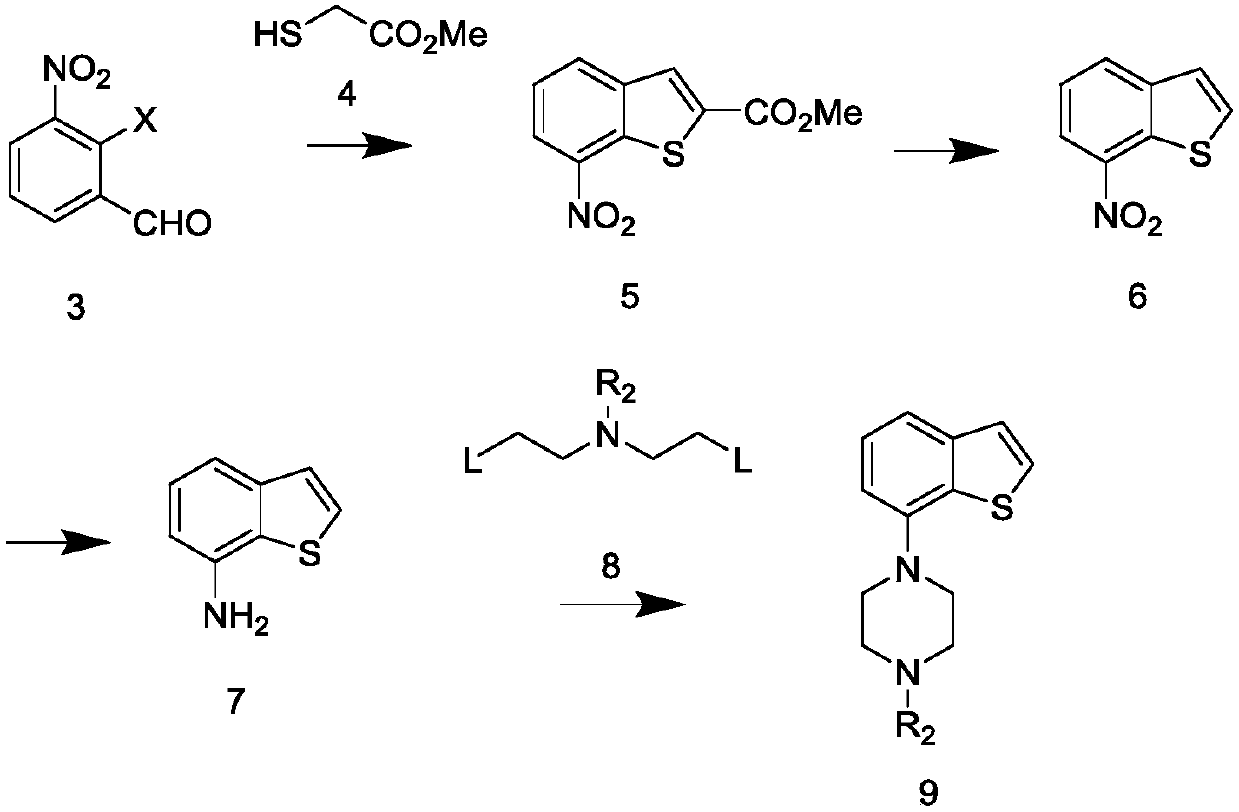
[0061] Combine 3.2 g of the solid obtained in Example 1 (compound 3-1, 0.01 mol), potassium carbonate (1.66 g, 0.012 mol, 1.2 eq), DMF (50 ml) under nitrogen protection, in an ice bath, and stir. After cooling to below 10 °C, methyl thioglycolate (1.17 g, 0.011 mol, 1.1 eq) was added. After the addition, the temperature was raised naturally, and the reaction was stirred for 6h. Sampling and testing, after the raw materials have reacted completely, pour the reaction solution into 150ml of ice water, stir for 15 minutes, a yellow solid precipitates out, filter with suction, rinse the filter cake with ice water, and dry to obtain 7-nitro-benzothiophene-2 Methyl carboxylate (compound 5), 2.3 g of yellow solid.
[0062] 1 H NMR (CDCl 3 )δ: 8.52 (1H, d, J = 8Hz), 8.23 (1H, d, J = 8Hz), 8.19 (1H, s), 7.62 (1H, t, J = 8Hz), 4.00 (3H, s)
[0063]
[0064] 2-Chloro-3-nitro-benzaldehyde (3.7g, 0.02mol), potassium carbonate (3.32g, 0.024mol, 1.2eq), DMF (70ml) were st...
Embodiment 3
[0066]
[0067] Two-step decarboxylation:
[0068] The 7-nitro-benzothiophene-2-carboxylic acid methyl ester (2.37g, 0.01mol) prepared by the preparation method of Example 2 was dissolved in methanol, and 1N aqueous sodium hydroxide solution (20ml, 2eq) was added, and the reaction was stirred for 2h . Sampling and testing showed that the reaction of the raw materials was complete. Spin to dry the solvent, dilute the residue with water, adjust the pH to 1~2 with 1N hydrochloric acid, extract with ethyl acetate, combine the organic phases, wash with saturated brine, dry over anhydrous sodium sulfate, filter with suction, and spin dry to obtain 7-nitro -Benzothiophene-2carboxylic acid, yellow solid 2.3g
[0069] The above-mentioned 7-nitro-benzothiophene-2 carboxylic acid (2.3g), DMF (20ml) and cuprous oxide (0.3g) were mixed and stirred, heated and reacted in an oil bath at 120°C for 2h, sampling was detected, the basic reaction was complete, and the reaction was reduced....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com