Method for preparing chiral alpha-unnatural amino acid by transition metal complex
A technology of unnatural amino acids and transition metals, which is applied in the field of preparation of α-unnatural amino acids, can solve the problems of long synthesis steps, difficulty in control, and limited scope of application, and achieve avoidance of post-processing, easy operation and amplification, and scope of application broad effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1S-(-6-tert-butoxycarbonyl)-alpha-(N-Fmoc-)-aminocaproic acid synthesis
[0052] The metal complexing agent in this embodiment is heterocyclic metal complexing agent 8-hydroxyquinoline, the halogenated hydrocarbon is aliphatic halogenated hydrocarbon tert-butyl bromobutyrate, and the transition metal is nickel. Proceed as follows:
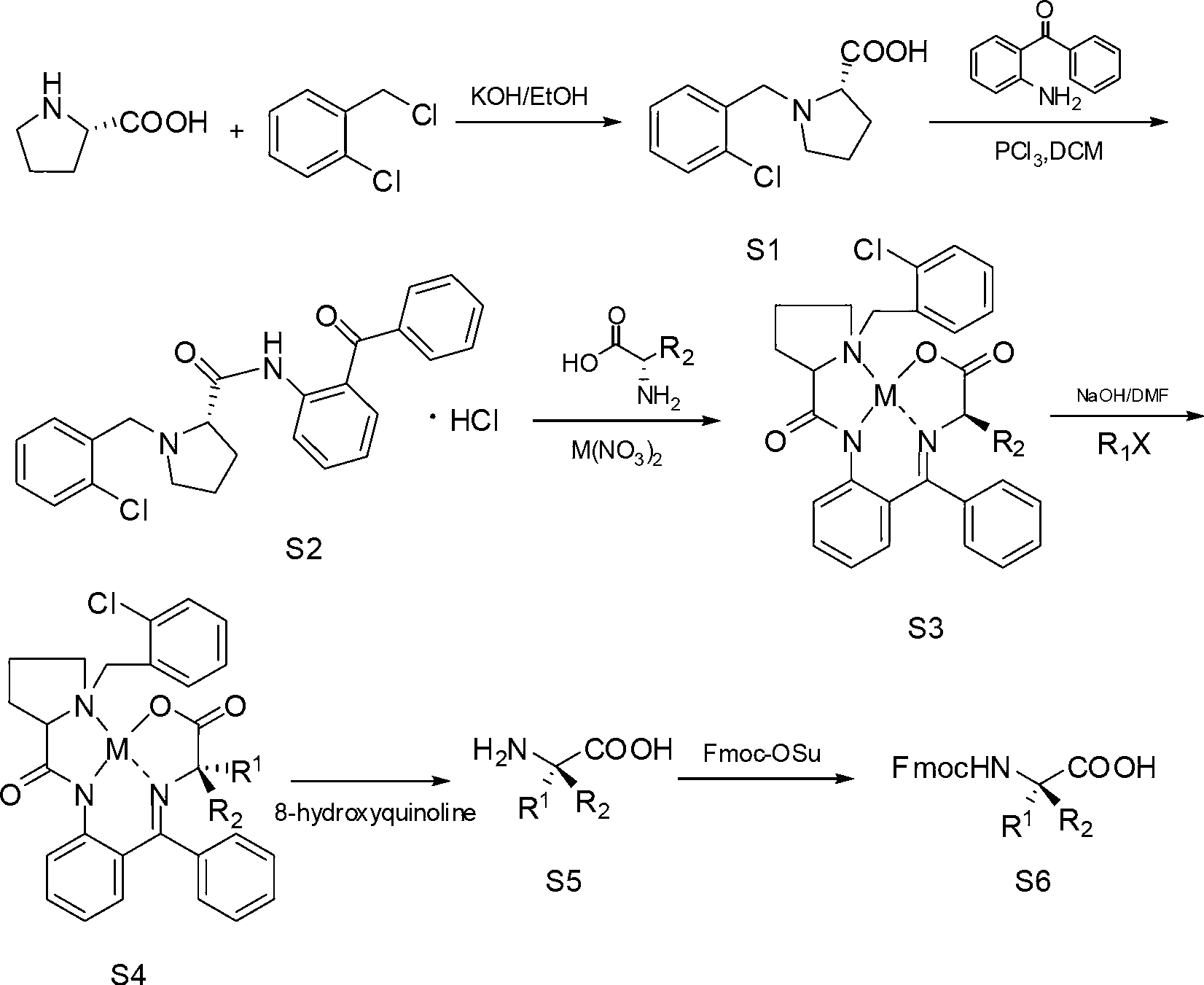
[0053] 1. Synthesis of S1
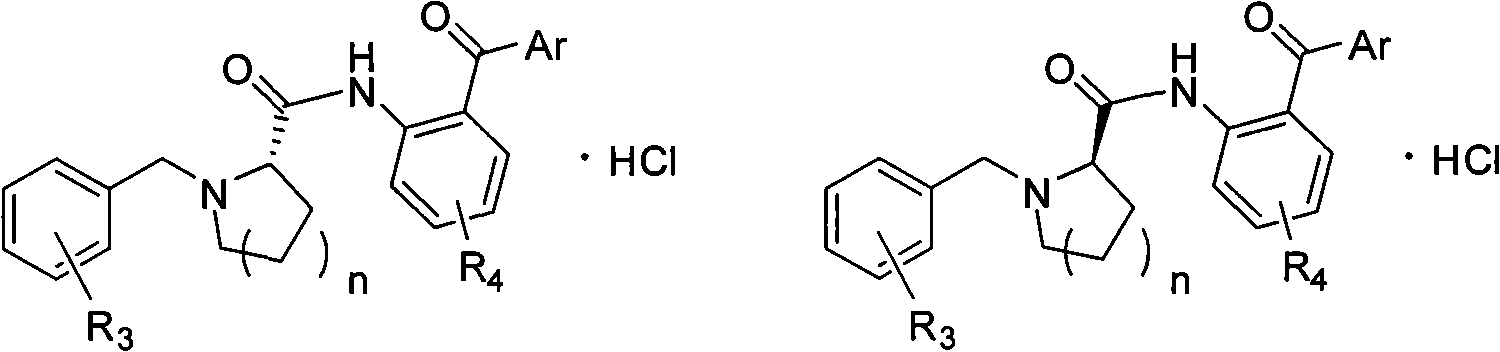
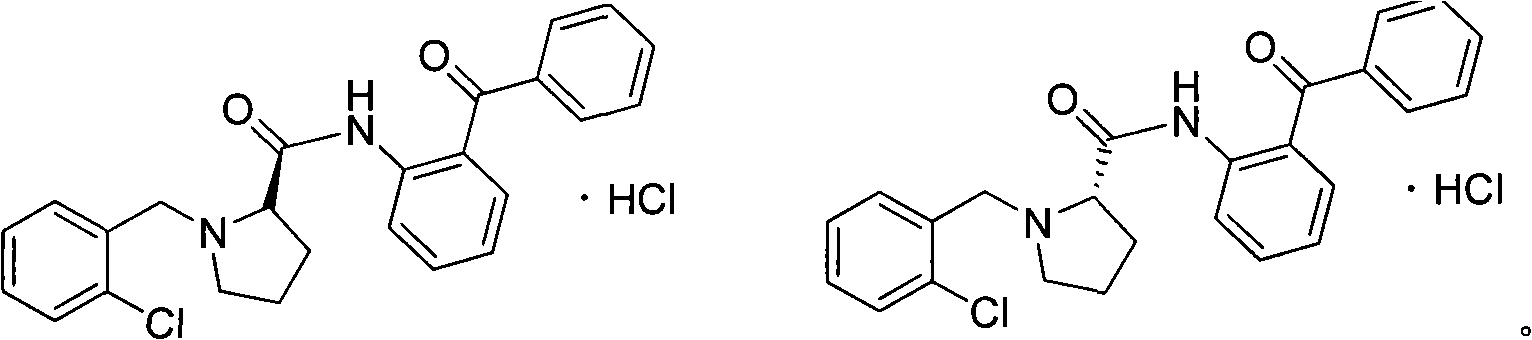
[0054] Heat 700mL of isopropanol to 55°C, add 145.9g of potassium hydroxide (2.6mol), after it is completely dissolved, add 100g of L-proline (0.87mol), add 167.5g of o-chlorobenzyl chloride (1.04mol) ); Stir, follow the reaction with thin layer chromatography until the L-proline reaction is complete, the reaction time is 18h; adjust the pH to 6 with 6mol / L hydrochloric acid, add dichloromethane and let stand for 4h; filter, filter cake with dichloromethane (DCM) was washed, and the combined filtrates were recrystallized with acetone after removing the solvent. 185.37 g of white solid S1 was obtained wi...
Embodiment 2
[0068] The synthesis of embodiment 2S-(-8-tert-butoxycarbonyl)-α-(N-Fmoc-)-aminocaprylic acid
[0069] In this embodiment, tert-butyl 6-bromobutyrate is used to replace tert-butyl 4-bromobutyrate in Example 1, and the metal complexing agent and transition metal are the same as in Example 1. The implementation steps of this embodiment are as follows:
[0070] 1.S1, S2, the synthetic method of S3 are identical with embodiment 1;
[0071] 2. Synthesis of S4
[0072] The synthesis method was the same as in Example 1, except that 18.06 g of tert-butyl 6-bromobutyrate (71.89 mmol) was used instead of tert-butyl 4-bromobutyrate in Example 1 to obtain 32 g of solid S4 with a yield of 65%.
[0073] 3. Synthesis of S5
[0074] Dissolve 70g S4 (0.1mol), 38.68g 8-hydroxyquinoline (0.226mol) in 1500mL acetonitrile, add 200mL H under stirring 2 O, keep the temperature at 30°C, stir and react for 12 hours; filter, wash the filter cake with water, combine the filtrates and evaporate aceto...
Embodiment 3
[0078] The synthesis of embodiment 3S-4-(2-ethoxyethoxy)-alpha-(N-Fmoc-)-aminobutyric acid
[0079] The metal complexing agent in this embodiment is ethylenediaminetetrapropionic acid, the halogenated hydrocarbon is 1-ethoxy-2-(2-iodoethoxy)ethane, and the transition metal is copper. Proceed as follows:
[0080] 1. The synthetic method of S1, S2 is identical with embodiment 1;
[0081] 2. Synthesis of S3
[0082] 25g S2 (0.055mol), 26.6g Cu(NO 3 ) 2 ·3H 2 O (0.11mol), 8.26g aminoacetic acid (0.11mol) was dissolved in 300mL methanol, and the methanol solution of KOH (25.3g KOH dissolved in 150mL methanol) was added dropwise under stirring, and the temperature of the system was kept below 50°C during the dropwise addition; After the addition is complete, heat to 60°C, stir and react for 3 hours (the reaction system changes from green to red); cool to room temperature, neutralize with 33g of acetic acid (55mol), adjust pH=6, distill off methanol, and remove the remaining Po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com