Method of synthesizing alkylated ferrocene derivative
A technology for alkylating ferrocene and ferrocene derivatives, applied in chemical instruments and methods, metallocenes, organic chemistry, etc., can solve problems such as large environmental impact and complicated process steps, and achieve high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
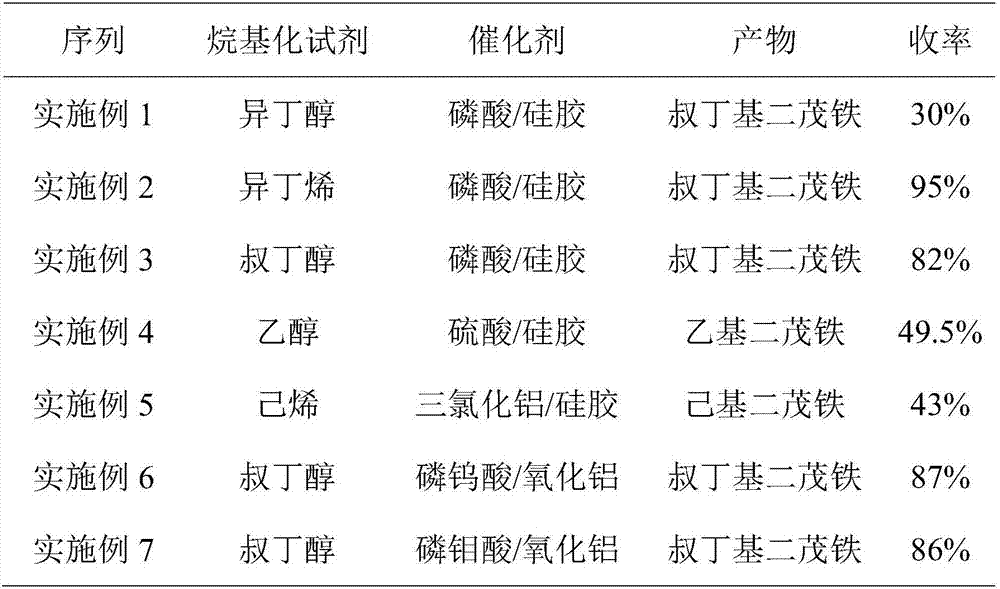
[0038] In this example, ferrocene is used as raw material, phosphoric acid supported on silica gel (loading capacity 25%) is used as catalyst, toluene is used as solvent, and isobutanol is used as alkylating agent.
[0039] Reactor volume: 100ml
[0040] Ferrocene: 2g
[0041] Catalyst: 1g
[0042] Alkylating agent: 4g
[0043] Toluene: 20ml
[0044] Reaction temperature: 120°C
[0045] Nitrogen pressure: 1MPa
[0046] Response time: 4h
[0047] Stirring rate: 500r / min
[0048] The reaction was carried out in a 100ml autoclave. Before the reaction, nitrogen gas was filled and discharged several times to replace the residual air in the still body, and 1MPa nitrogen gas was charged to maintain the high-pressure environment in the still. Stir and raise to the reaction temperature of 120°C at a rate of 5°C / min. After reacting for 4 hours, stop heating, slowly release nitrogen after cooling, and filter to obtain the filtrate as the mixture of raw materials and products. Th...
Embodiment 2
[0051] In this example, ferrocene is used as raw material, phosphoric acid supported on silica gel (loading capacity 50%) is used as catalyst, trimethylbenzene is used as solvent, and isobutylene is used as alkylating agent.
[0052] Reactor volume: 100ml
[0053] Ferrocene: 2g
[0054] Catalyst: 1g
[0055] Alkylating agent: 8g
[0056] Trimethylbenzene: 20ml
[0057] Reaction temperature: 180°C
[0058] Nitrogen pressure: 1MPa
[0059] Response time: 4h
[0060] Stirring rate: 500r / min
[0061] The reaction was carried out in a 100ml autoclave. Before the reaction, nitrogen gas was filled and discharged several times to replace the residual air in the still body, and 1MPa nitrogen gas was charged to maintain the high-pressure environment in the still. Stir and raise to a reaction temperature of 180°C at a rate of 5°C / min. After reacting for 4 hours, stop heating, slowly release nitrogen after cooling, and filter to obtain the filtrate as the mixture of raw materials ...
Embodiment 3
[0064] In this example, ferrocene is used as raw material, phosphoric acid supported on silica gel (loading capacity 75%) is used as catalyst, toluene is used as solvent, and tert-butanol is used as alkylating agent.
[0065] Reactor volume: 100ml
[0066] Ferrocene: 2g
[0067] Catalyst: 1g
[0068] Alkylating agent: 2g
[0069] Toluene: 20ml
[0070] Reaction temperature: 230°C
[0071] Nitrogen pressure: 1MPa
[0072] Response time: 4h
[0073] Stirring rate: 500r / min
[0074] The reaction was carried out in a 100ml autoclave. Before the reaction, nitrogen gas was filled and discharged several times to replace the residual air in the still body, and 1MPa nitrogen gas was charged to maintain the high-pressure environment in the still. Stir and raise to reaction temperature 230°C at a rate of 5°C / min. After reacting for 4 hours, stop heating, slowly release nitrogen after cooling, and filter to obtain the filtrate as the mixture of raw materials and products. The mix...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com