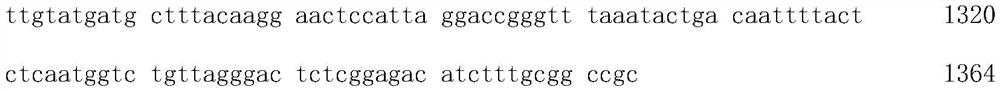Preparation and application of Pichia pastoris whole-cell catalyst expressing Candida tropicalis lipase intracellularly
A recombinant strain, typical technology, applied in the field of bioengineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Preparation of Pichia pastoris whole-cell catalyst expressing Candida tropicalis lipase intracellularly:
[0022] According to the codon preference of Pichia pastoris, the lipase gene of Candida tropicalis was codon-optimized, and then the codon-optimized lipase gene of Candida tropicalis was synthesized in vitro. The gene contains KpnI and NotI restriction sites. The gene and the pPICZB plasmid were digested with KpnI and NotI respectively, and then the digested products were ligated, and the obtained recombinant plasmid was transformed into E.coli Top 10 competent cells, and recombinants containing the recombinant plasmid were screened.
[0023] The recombinant plasmid was extracted from the recombinant, and SalI linearized the recombinant plasmid at 37°C for 2 hours, and then transferred the recombinant plasmid into Pichia pastoris GS11 by electroporation, and the electroporation conditions were 1500V, 25μF, 200Ω. Add 1mL 1mol / L sorbitol immediately after the electr...
Embodiment 2
[0028] The Pichia pastoris whole-cell catalyst expressing Candida tropicalis lipase prepared in Example 1 catalyzes castanospermine and vinyl butyrate to synthesize 6-O-butyryl castanospermine:
[0029] Add 4 ml of THF, 4 micromol of castanospermine, 8 micromol of vinyl butyrate and 100 mg of Pichia whole-cell catalyst expressing Candida tropicalis lipase in a 10 ml stoppered Erlenmeyer flask. The yield of 6-O-butyryl castanospermine reached 27.2% within 60 minutes under the conditions of 30°C and 160r / min.
[0030] Codon-optimized Candida tropicalis lipase gene sequence:
[0031] Jiangxi Normal University
[0032] Preparation and Application of Pichia pastoris Whole Cell Catalyst Expressing Candida tropicalis Lipase Intracellularly
[0033] 1
[0034] Patent In Version 3.5
[0035] 1
[0036] 1364
[0037] DNA
[0038] Artificial sequence
[0039]
[0040] 1
[0041]
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com