A kind of preparation method of composite visible light catalyst
A catalyst and visible light technology, applied in the field of materials science, can solve the problems of complex preparation process, poor chemical stability, low quantum yield, etc., and achieve the effect of simple preparation method, low cost and good photocatalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1), according to the elemental P and AgNO 3 Weigh P (0.001g, 0.033mmol) and AgNO with a molar ratio of 1:3 3 (0.017g, 0.1mmol);
[0025] (2), according to Zr 4+ with Ag + Weigh ZrCl at a molar ratio of 10:1 4 (0.233g, 1mmol), according to ZrCl 4 Weigh terephthalic acid (0.199g, 1.1mmol) at a molar ratio of 1.2:1;
[0026] (3) Disperse the raw materials in step (1) (2) into 20ml of N,N-dimethylformamide solvent, and at the same time, according to the volume ratio of N,N-dimethylformamide and acetic acid is 12:1 Add 1.7ml of acetic acid in the ratio of 1.7ml, stir at room temperature for 20min, then transfer to a polytetrafluoroethylene-lined reactor, control the reaction temperature to 180°C, the time is 24h, cool at room temperature, filter, and dry to obtain Ag 3 PO 4 / UiO-66 composite visible light catalyst, denoted as Ag 3 PO 4 / UiO-66-10.
Embodiment 2
[0028] (1), according to the elemental P and AgNO 3 Weigh P (0.002g, 0.067mmol) and AgNO with a molar ratio of 1:3 3 (0.034g, 0.2mmol);
[0029] (2), according to Zr4+ with Ag + Weigh ZrCl at a molar ratio of 5:1 4 (0.233g, 1mmol), according to ZrCl 4 Weigh terephthalic acid (0.183g, 1.1mmol) at a molar ratio of 1.1:1;
[0030] (3) Disperse the raw materials in step (1) (2) into 20ml of N,N-dimethylformamide solvent, and at the same time, according to the volume ratio of N,N-dimethylformamide and acetic acid is 11:1 Add 1.8ml of acetic acid in the ratio of 1.8ml, stir at room temperature for 15min, then transfer to a polytetrafluoroethylene-lined reactor, control the reaction temperature to 170°C, the time is 22h, cool at room temperature, filter, and dry to obtain Ag 3 PO 4 / UiO-66 composite visible light catalyst, denoted as Ag 3 PO 4 / UiO-66-5.
Embodiment 3
[0032] (1), according to the elemental P and AgNO 3 Weigh P (0.01g, 0.33mmol) and AgNO with a molar ratio of 1:3 3 (0.17g, 1mmol);
[0033] (2), according to Zr 4+ with Ag + The molar ratio of ZrCl is 1:1. 4 (0.233g, 1mmol), according to ZrCl 4 Weigh terephthalic acid (0.166g, 1mmol) at a molar ratio of 1:1;
[0034] (3) Disperse the raw materials in step (1) (2) into 20ml of N,N-dimethylformamide solvent, and at the same time, according to the volume ratio of N,N-dimethylformamide and acetic acid is 10:1 Add 2ml of acetic acid in the proportion of acetic acid, stir at room temperature for 10min, then transfer to a polytetrafluoroethylene-lined reactor, control the reaction temperature to 160°C, the time is 20h, cool at room temperature, filter, and dry to obtain Ag 3 PO 4 / UiO-66 composite visible light catalyst, denoted as Ag 3 PO 4 / UiO-66-1.
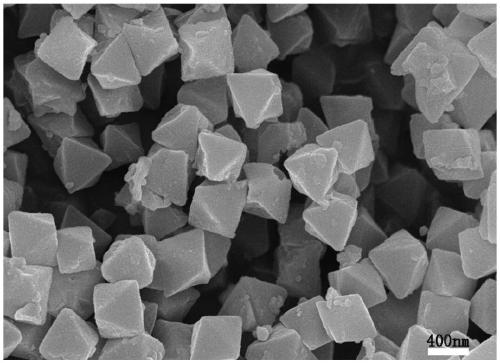
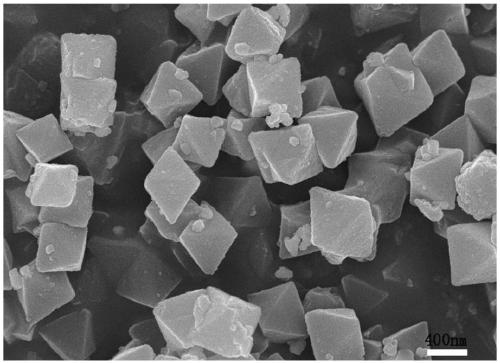
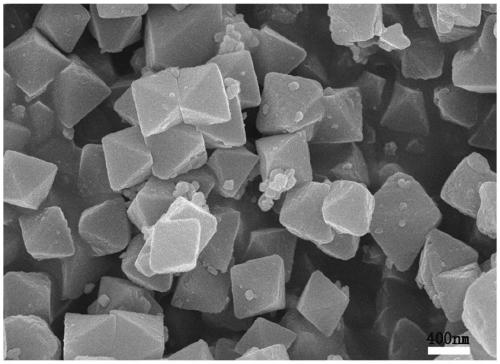
[0035] Depend on figure 1 , 2 , 3. It can be seen that the synthesized Uio-66 has a regular octahedral structure, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com