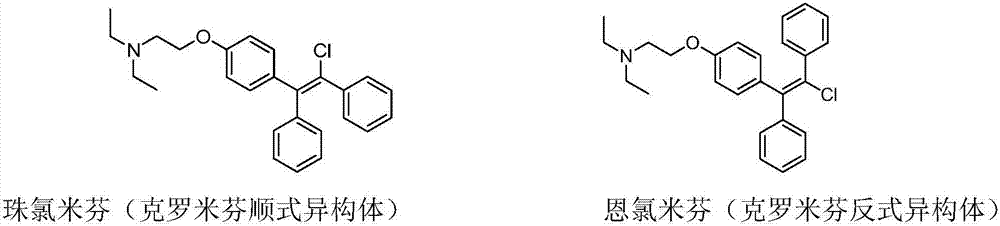
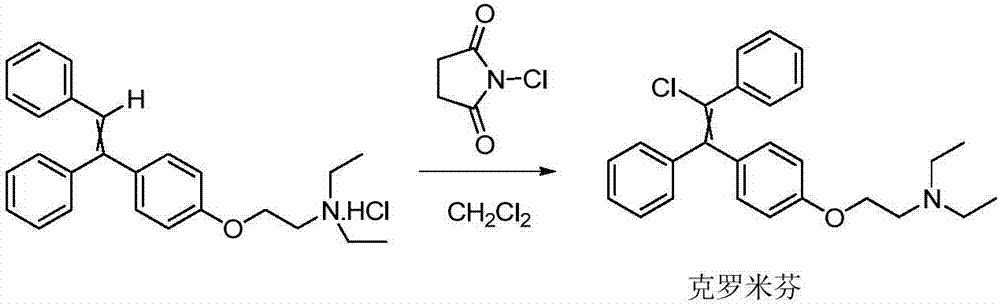
Preparation method of enclomiphene
A technology of enclomiphene and mixed solvent is applied in the field of preparation of enclomid, can solve the problems of unsuitability for industrial production, complicated operation process, low splitting efficiency, etc., achieves good industrial application prospect, simple operation, easy operation and the like. The effect of recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: the preparation of Clomiphene cis-trans isomer mixture
[0057]
[0058] N,N-diethyl-2-[4-(1,2-diphenylethenyl)phenoxy]ethylamine hydrochloride (59.8g, content 92%) was dissolved in 730mL of dichloromethane, After adding 20.35 g of N-chlorosuccinimide at one time, it was heated to reflux and reacted in the dark for 6 hours. HPLC monitors that the reaction of raw materials is complete. In HPLC, the cis peak area ratio is 24.50%, and the trans peak area ratio is 61.26%. The reaction solution is washed twice with 436 ml of excess 2% sodium hydroxide aqueous solution, washed with water, washed with saturated saline, and dried. After concentration, 50.0 g of a mixture of cis and trans isomers of clomiphene was obtained, with a purity of 85.76% and a crude yield of 78.2%.
Embodiment 2
[0059] Embodiment 2: Preparation of cis-trans isomer mixture of clomiphene citrate
[0060] Dissolve 25.0 g of the cis-trans isomer mixture of clomiphene obtained in Example 1 in 25 mL of absolute ethanol, add 12.18 g of anhydrous citric acid, heat up to reflux reaction for 5 minutes after the addition, and cool down naturally until the room temperature is 20°C No solid precipitated, continued to stir for half an hour after cooling in an ice bath, precipitated a white viscous solid, added 50 mL of tert-butyl methyl ether to dilute, continued to stir in an ice bath for half an hour, filtered, washed the solid with a small amount of tert-butyl methyl ether, and dried to obtain clomiphene citrate Salt 30.8g, yield 97.53%.
Embodiment 3
[0061] Embodiment 3: the preparation of clomiphene malonate cis-trans isomer mixture
[0062] With reference to the method of Example 2, 12.6 g of the corresponding cis-trans isomer mixture of clomiphene malonate was prepared, with a yield of 93.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com