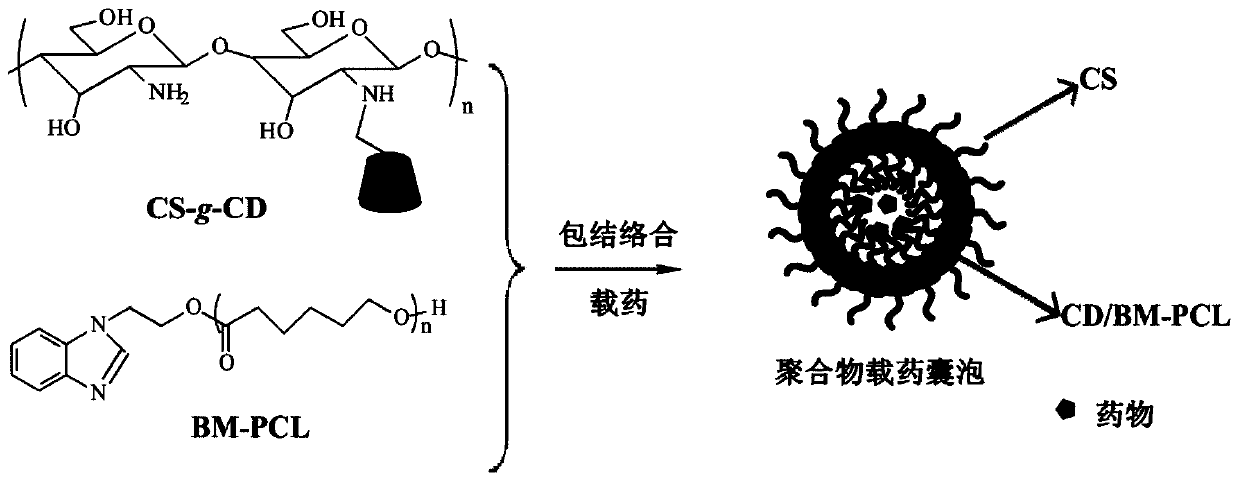
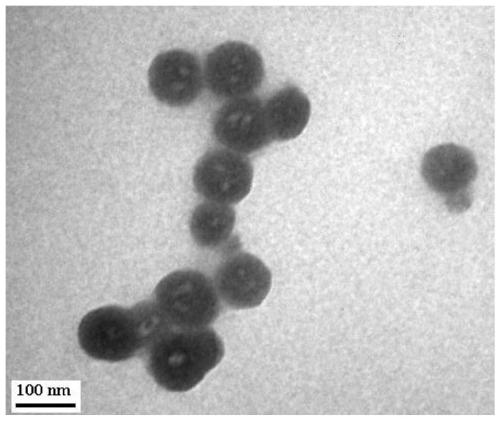
A kind of preparation method of cyclodextrin polymer drug-loaded vesicle
A cyclodextrin polymer and drug-carrying technology, which is used in pharmaceutical formulations, non-active components of polymer compounds, and medical preparations with non-active components, etc. Low problems, to achieve the effect of strong drug-carrying capacity, simple preparation process, easy operation and realization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A preparation method of cyclodextrin polymer drug-loaded vesicles, comprising the steps of:
[0038] 1) Dissolve CS-g-CD in 10ml of 1% (v / v) glacial acetic acid solution (pH=3) to prepare a solution with a concentration of 1mg / mL, and dissolve BM-PCL in 10ml of DMF to prepare A solution with a concentration of 0.1 mg / mL was obtained;
[0039] 2) Add 6.7 mg of doxorubicin to the BM-PCL solution and stir to dissolve, then add it dropwise to the CS-g-CD solution at a rate of 1-2 drops / s under stirring conditions, and continue stirring after the addition is complete After 24 hours, dialyze in distilled water for 6 hours to obtain polymer drug-loaded vesicles with a drug loading capacity of 37.8%.
Embodiment 2
[0041] A preparation method of cyclodextrin polymer drug-loaded vesicles, comprising the steps of:
[0042] 1) Dissolve CS-g-CD in 20ml of 1% (v / v) glacial acetic acid solution (pH=3) to make a solution with a concentration of 0.1mg / mL, and dissolve BM-PCL in 5ml of DMF Prepare a solution with a concentration of 0.1 mg / mL;
[0043] 2) Add 1.7 mg of naproxen to the BM-PCL solution and stir to dissolve, then add dropwise to the CS-g-CD solution at a rate of 1-2 drops / s under stirring conditions and mix, continue to Stir for 48 hours and dialyze in distilled water for 4 hours to obtain polymer drug-loaded vesicles with a drug loading capacity of 39.8%.
Embodiment 3
[0045] A preparation method of cyclodextrin polymer drug-loaded vesicles, comprising the steps of:
[0046] 1) Dissolve CS-g-CD in 50ml of 1% (v / v) glacial acetic acid solution to make a solution with a concentration of 0.1mg / mL, and dissolve BM-PCL in 5ml of DMF to make a concentration of 0.5 mg / mL solution;
[0047] 2) Add 10.3 mg of indomethacin into the BM-PCL solution and stir to dissolve, and then add it dropwise to the CS-g-CD solution at a rate of 1-2 drops / s under stirring conditions and mix. Stirring was continued for 12 hours and dialyzed in distilled water for 8 hours to obtain polymer drug-loaded vesicles with a drug loading capacity of 37.1%.
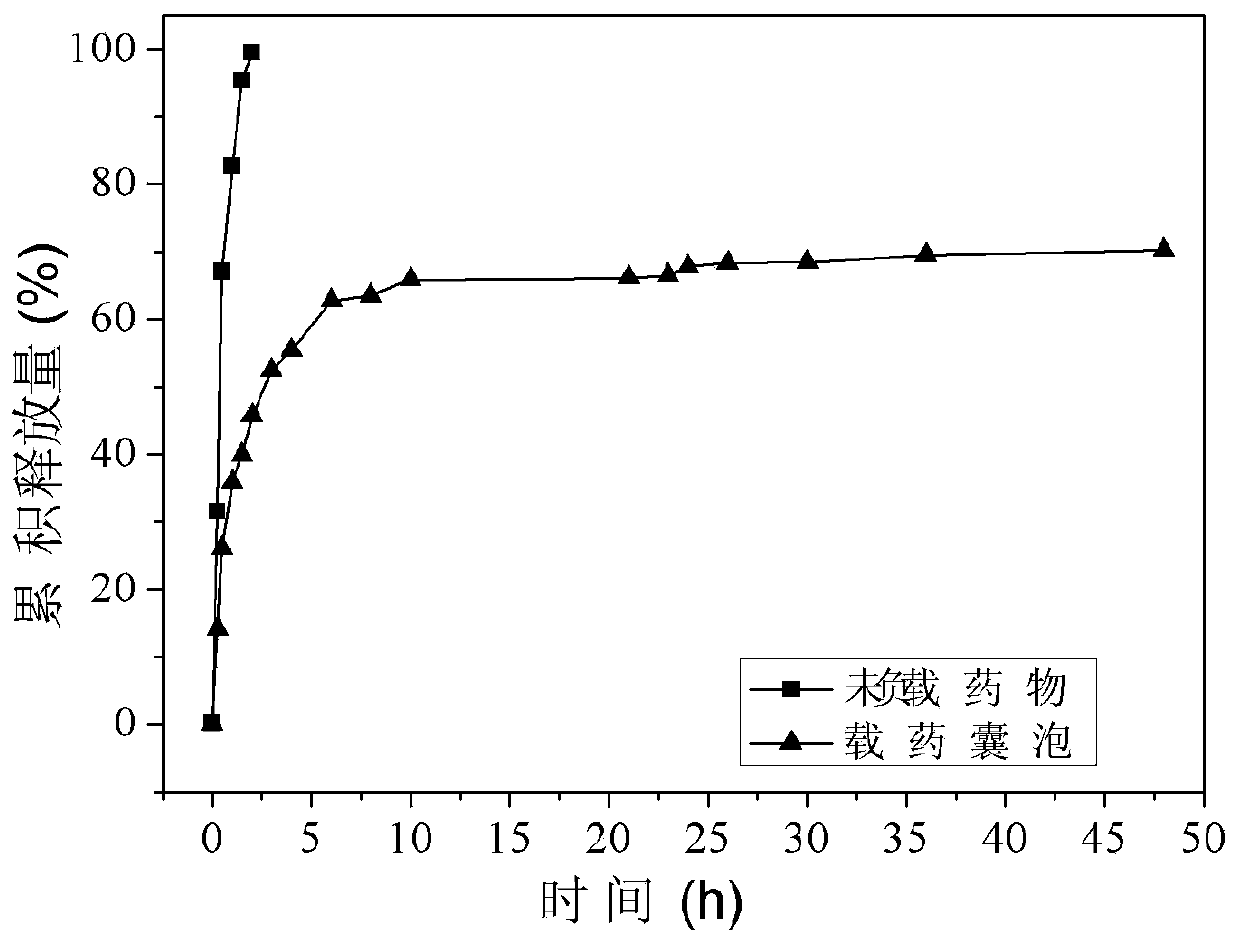
[0048]In order to verify the drug controlled release and sustained release effect of the polymer drug-loaded vesicle obtained in the present invention, we have carried out the test of the controlled release and sustained release effect of the drug, and the specific results are as follows image 3 Shown:
[0049] Depend ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com