Preparation method and application of conductive hydrogel capable of slowly releasing drugs and factors
A conductive hydrogel and sustained-release drug technology, which can be used in pharmaceutical formulations, drug delivery, medical preparations with inactive ingredients, etc. Increase the effect of immobilizing bioactive growth factors, stabilizing metal-organic framework structures, and improving electrochemical activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
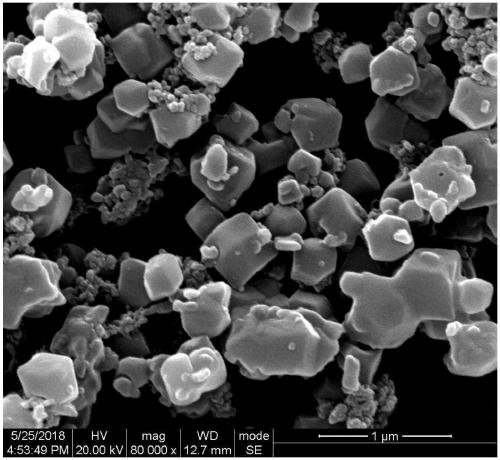
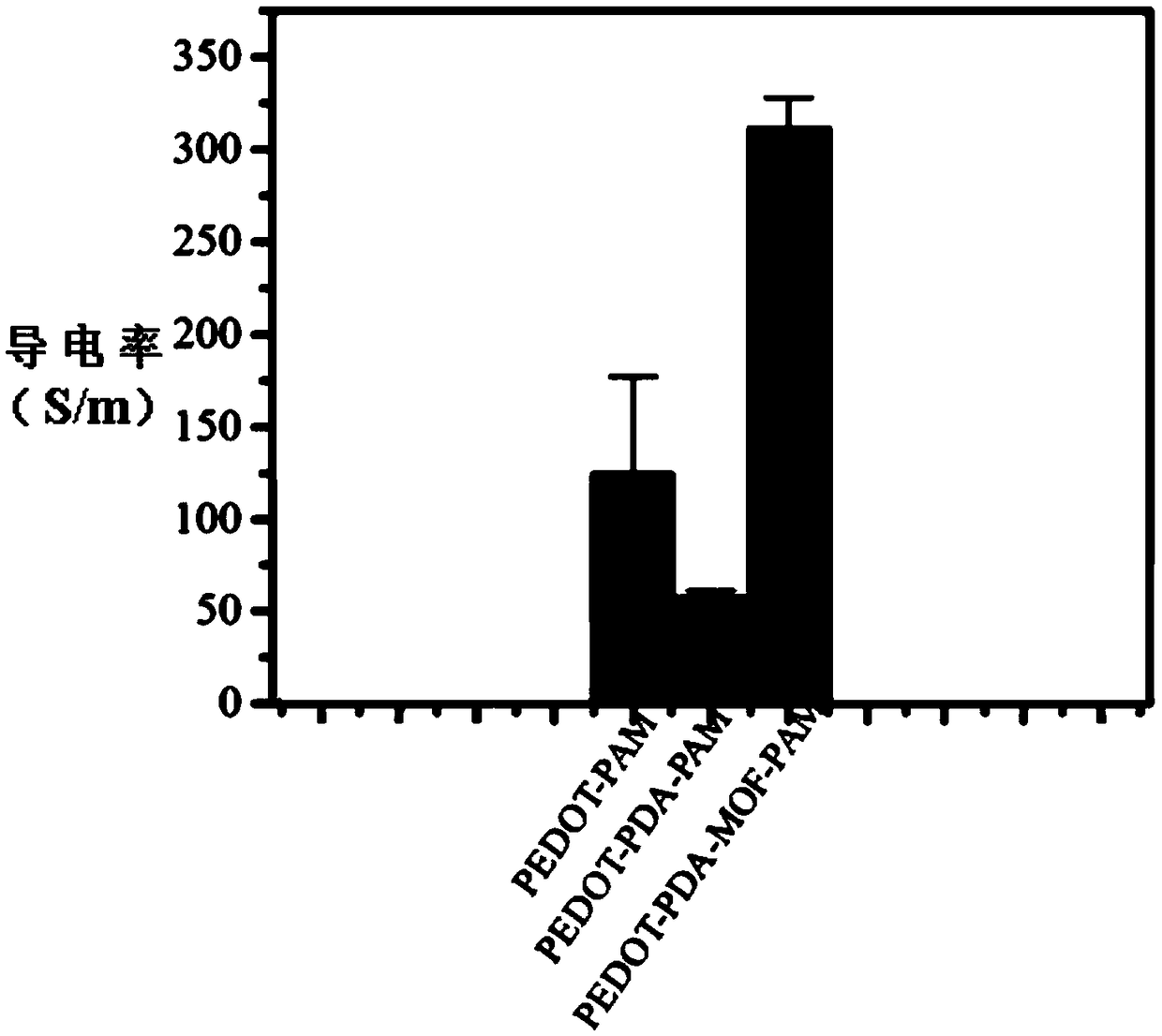
Image
Examples
preparation example Construction
[0027] The preparation method of the conductive hydrogel capable of sustained release of medicines and factors comprises the following steps:
[0028] Step 1: Prepare a conductive polymer monomer solution with a mass concentration of 0.04-2%, add an oxidizing agent, stir and react at 0-5°C for 4-10 days, perform centrifugal cleaning several times after sufficient reaction, and obtain a conductive polymer polymer material; the mass ratio of the conductive polymer monomer to the oxidizing agent is 1:1~20; the oxidizing agent is one of ferric chloride, ferric nitrate, ferric sulfate and ammonium ferric sulfate; the conductive polymer monomer is pyrrole monomer, One of aniline monomers, thiophene monomers and 5-carboxyindole monomers.
[0029] Step 2: Prepare a dopamine aqueous solution with a concentration of 0.05-10 mg / mL, adjust the pH to 8-13 with ammonia water or trisaminomethane buffer solution; add the conductive polymer obtained in step 1, and stir at room temperature for ...
Embodiment 1
[0039] A preparation method of conductive hydrogel, comprising the following steps:
[0040] A, preparation of polydopamine / poly 3,4-ethylenedioxythiophene complex
[0041]Dissolve 266 μL of 3,4-ethylenedioxythiophene monomer in 30 mL of absolute ethanol to obtain a solution with a mass concentration of 1.5% of 3,4-ethylenedioxythiophene monomer; add 7 g of trichloride to the above solution iron, stirred and reacted at 0-5°C for 5 days; the solution after the above reaction was centrifuged and washed several times with deionized water to obtain poly-3,4-ethylenedioxythiophene particles; dopamine was dissolved in deionized water to obtain A dopamine solution with a concentration of 0.5 mg / mL, the pH of the above solution was adjusted to 8.5 with ammonia water; then, poly-3,4-ethylenedioxythiophene particles were added to the above solution, stirred at room temperature for 12 hours, and washed several times to obtain poly Dopamine / poly-3,4-ethylenedioxythiophene complex.
[00...
Embodiment 2
[0050] A, preparation of polydopamine / polypyrrole complex
[0051] Dissolve 190 μL of pyrrole monomer in 40 mL of absolute ethanol to obtain a solution with a mass concentration of 0.58% pyrrole monomer; add 5 g of iron sulfate to the above solution, and stir and react at 0-5°C for 3 days; The final solution was centrifuged and washed 3 times with deionized water to obtain polypyrrole particles; dopamine was dissolved in deionized water to obtain a solution with a dopamine concentration of 0.4 mg / mL, and the pH of the above solution was adjusted to 8.5 with trisaminomethane buffer. ; Then, add polypyrrole particles to the above solution, stir at room temperature for 12 hours, and wash several times to obtain a polydopamine / polypyrrole complex.
[0052] B, Preparation of ZIF-7 / polydopamine / polypyrrole complex
[0053] Dissolve zinc acetate in N,N-dimethylformamide to obtain a solution with a mass concentration of zinc acetate of 2.15%; dissolve benzimidazole in methanol to obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com