(+/-) Uncarilins A and B, and pharmaceutical composition and application thereof
A composition and drug technology, applied in the field of medicine, can solve the problems of no compound pharmaceutical composition, no compound, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
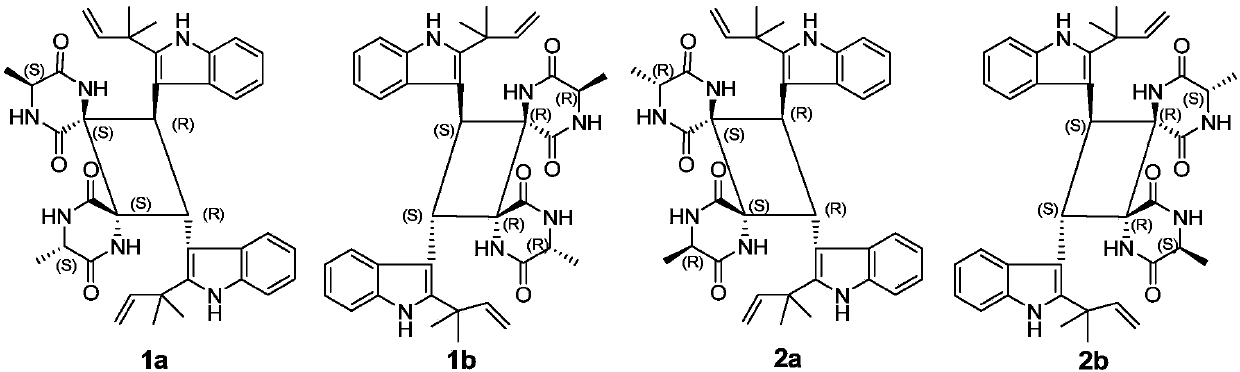
[0025] Preparation of Compounds 1a / 1b and 2a / 2b:
[0026] Take the dried hooked stems and branches of Uncaria uncaria, crush them, extract twice with 90% ethanol under reflux, each time for 3 hours, combine the ethanol extracts, recover the ethanol under reduced pressure to obtain the extract. The extract was dissolved and adsorbed on silica gel with 80% ethanol, placed at room temperature to evaporate the solvent, ground and sieved, and subjected to silica gel column chromatography, followed by gradient elution with chloroform and chloroform-methanol (9:1). The fraction eluted with chloroform-methanol (9:1) was further subjected to silica gel column chromatography and eluted isocratically with petroleum ether-acetone (1:1) to obtain 4 components A-D. Component C was prepared by MCI CHP-20Pgel column under medium pressure, and eluted with acetonitrile-water gradient from 20:80 to 80:20 to obtain 5 fractions C-1-C-5. C‐2 was purified by sephadexLH‐20 column chromatography, elu...
Embodiment 2
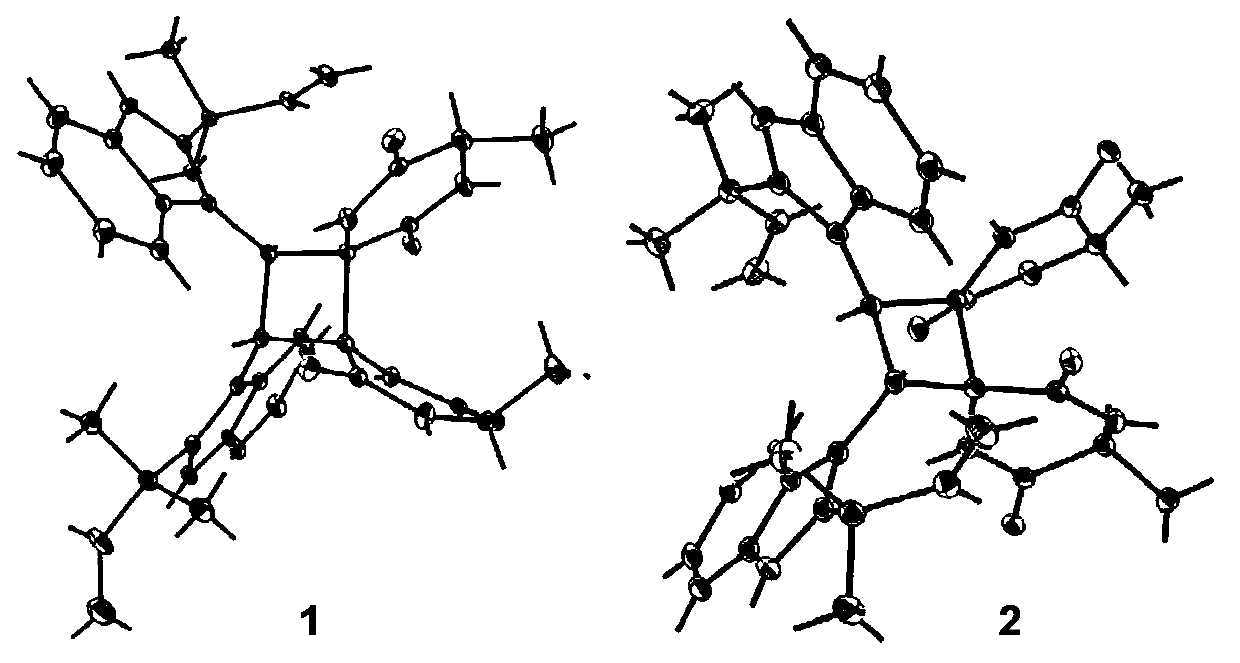
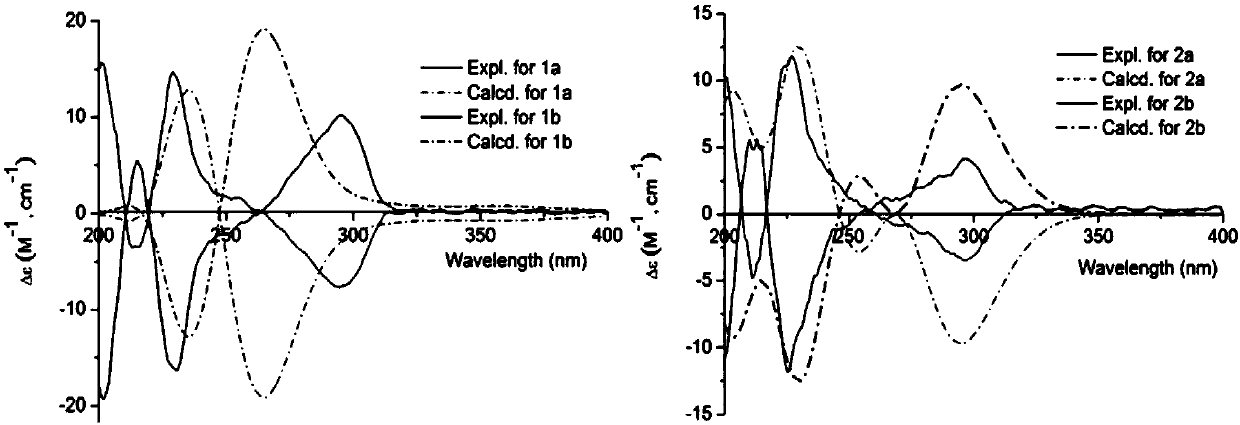
[0028] Structural identification of compounds 1a / 1b and 2a / 2b:
[0029] Optical rotation was measured by Jascommodel 1020 polarimeter (Horiba, Tokyo, Japan); infrared spectrum (IR) was measured by KBr pellet method, and was measured by Bio-Rad FTS-135 infrared spectrometer (Hercules, California, USA); ultraviolet spectrum was measured by UV- 2401PC type ultraviolet spectrometer (Shimadzu, Kyoto, Japan) was measured; ECD spectrum was measured by Applied Photophysics circular dichroism spectrometer (Agilent, Santa Clara, United States); nuclear magnetic resonance spectrum (1D and 2D NMR) was measured by AVANCE III‐600 superconductor Nuclear magnetic resonance (Bruker, Bremerhaven, Germany) was used to measure deuterated methanol as solvent and TMS (tetramethylsilane) as internal standard; high-resolution mass spectrometry (HRMS) was performed with LCMS‐IT‐TOF mass spectrometer (Shimadzu, Kyoto, Japan). Japan) determination; TLC silica gel and column chromatography silica gel (20...
Embodiment 3
[0056] Compounds 1a / 1b and 2a / 2b on MT 1 and MT 2 Agonistic activity of the receptor.
[0057] 1 Materials and methods
[0058] 1.1 Materials:
[0059] MT 1 and MT 2 The cell lines used for activity screening correspond to human kidney epithelial cells HEK293‐MT 1 and HEK293‐MT 2 ; Cell culture medium (Dulbecco's Modified Eagle Medium, DMEM) containing 10% fetal bovine serum; Wash-free calcium flow kit.
[0060] 1.2 Instrument: CO 2 Constant temperature incubator Thermo Forma 3310 (USA); Inverted biological microscope XD‐101 (Nanjing); Flexstation 3 Benchtop Multi‐Mode Microplate Reader (MolecularDevices, Sunnyvale, California, USA).
[0061] 1.3 Experimental process
[0062] Spread the matrix BD Matrigel on the 96-well black-walled transparent bottom plate, and after 1 hour in a 37°C constant temperature incubator, draw the supernatant and divide it into 4×10 4 / well density, the corresponding cells were seeded in a 96-well black-walled transparent bottom plate, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com