Methods and devices relating to the detection of oral cancer biomarkers
A biomarker and oral cancer technology, applied in biochemical cleaning devices, biochemical equipment and methods, enzymology/microbiology devices, etc., can solve the problem of not providing oral sample device OSCC
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Direct measurement of interleukin (IL) from a solid support
[0037] Recombinant IL-2±vector (R & D Systems; Cat. 202-IL-CF-10 μg; lot AE4309112 and Cat. 202-IL-10 μg; lot AE4309081, respectively) was dissolved in blood at 50 pg or 100 pg / μl (TCS Biosciences). Aliquots (1 μl containing 50 (B) or 100 (A) pg IL-2) were applied to Whatman 903 filter paper.
[0038] The samples were allowed to dry overnight at ambient temperature and humidity. Using an appropriately sized punch, a 3 mm diameter punched disk was extracted from each paper type. Individual discs were directly assayed for IL-2 using reagents from the complete 10-configuration IL-2 QuantikineELISA kit (R & D Systems, Cat. D2050, lot 273275). Direct assays are performed "punch in well", ie, where sections 903 of filter paper are punched out and deposited in reaction wells of conventional multiwell plates.
[0039] Upon completion of the assay, the optical density was monitored at 450 nm. IL-2 reco...
Embodiment 2
[0040] Example 2: Model enzyme assays from cells or enzymes transferred to solid supports
[0041] Protein and enzyme assays were performed using fully configured DNase and RNase Contamination kits (DNase & RNAase Alert QC Systems, cat# AM1970 & AM1966, Life Technologies) according to the manufacturer's instructions.
[0042] Dideoxyribonuclease (DNase)
[0043] In the first series of experiments, 0.125-0.5 U DNase was applied to Whatman FTA and 903 paper in a volume of 10 µl. DNase and RNase activities were measured as outlined below.
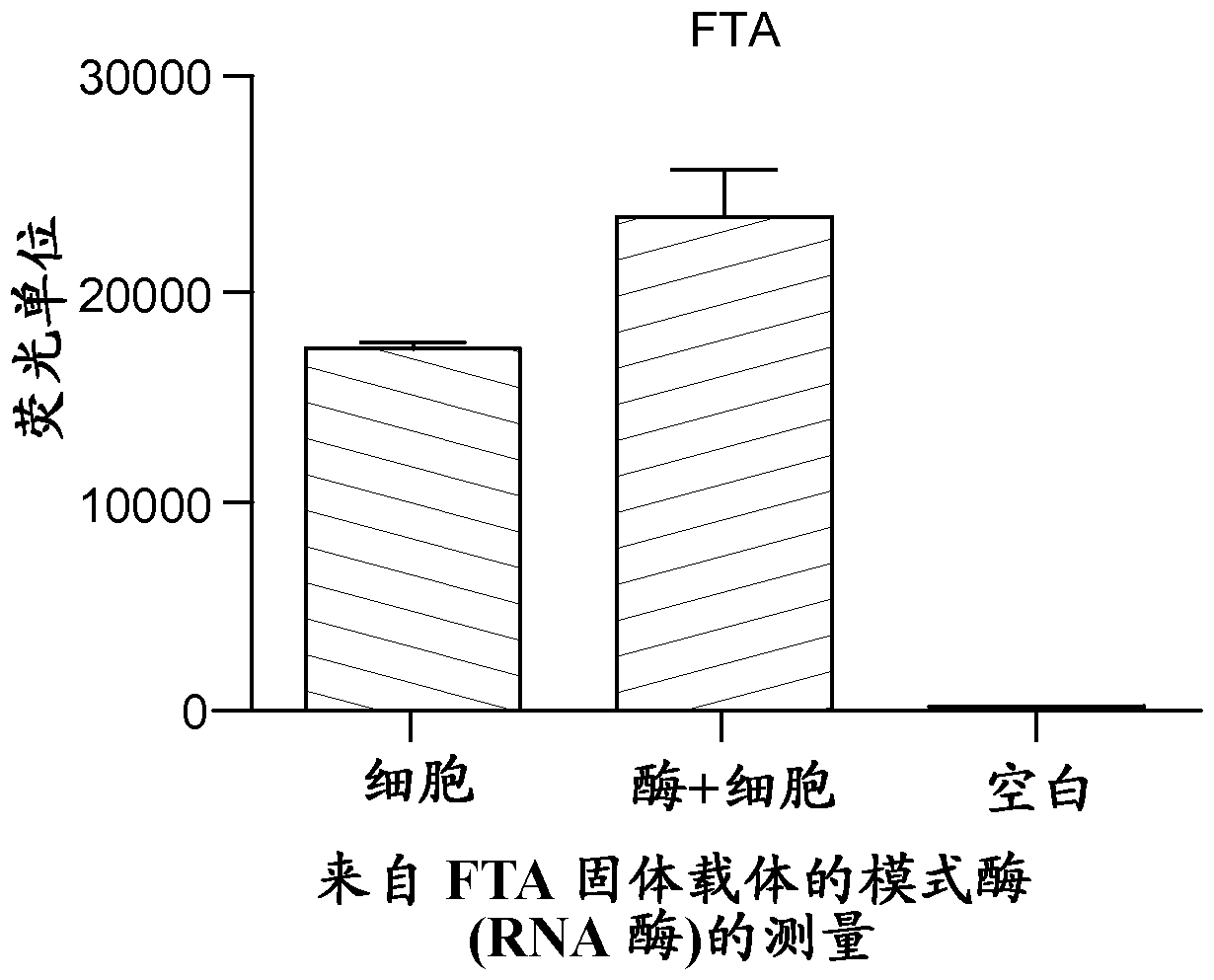
[0044] In a second series of experiments, 1.2 mm punches were taken from 106 human embryonic stem cells (GE Healthcare; cell line ref: WCB307 GEHC 28) that had been applied to FTA and 903 paper in a volume of 10 μl as above. DNase and RNase activities were measured as outlined below.
[0045] In a third series of experiments, from 106 human embryonic stem cells (GEHealthcare; cell line ref: WCB307 GEHC 28) that had been applied to FTA and...
Embodiment 3
[0049] Example 3: Measuring Lactate Dehydrogenase (LDH)
[0051] LDH is a cytosolic enzyme present in many different types of cells. Upon injury to the plasma membrane, LDH is released into the bathing medium surrounding the cell. Released LDH can be quantified by coupled enzyme reactions. First, LDH catalyzes the conversion of lactate to pyruvate by reducing NAD+ to NADH. Second, diaphorase uses NADH to reduce tetrazolium salt (INT) to red formazan product. Therefore, A The level produced is directly proportional to the amount of LDH released in the medium. Assays were performed by transferring the punch and adding cells or LDH enzyme or into a microplate and adding kit reagents (LDH Cytotoxicity Assay Kit; Thermo Scientific, Product Code 88953). After incubation for 30 minutes at room temperature, the reaction was stopped and LDH activity was determined by spectrophotometric absorbance at 490 nm.
[0052] Immunoassay for LDH
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com