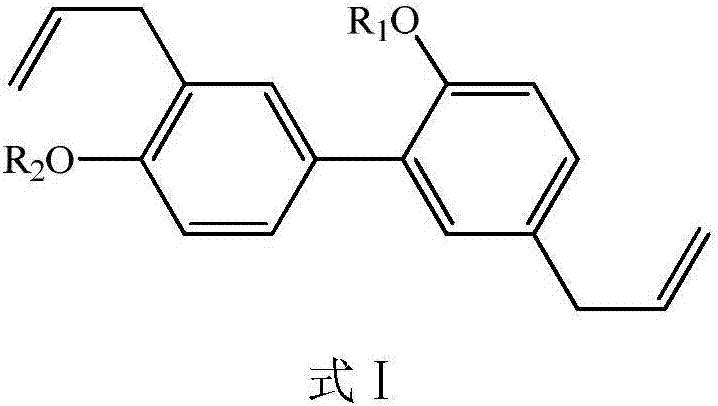
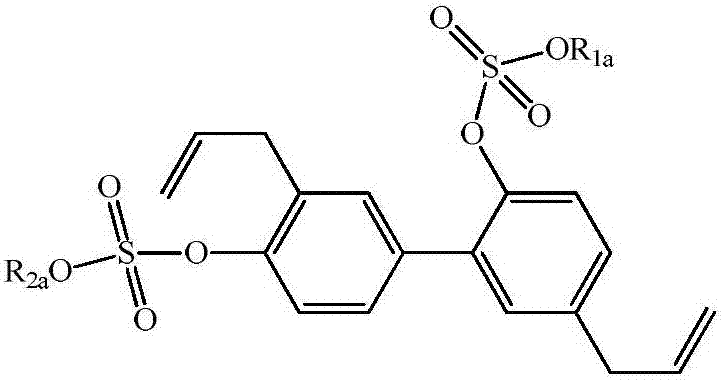
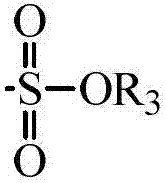Honokiol derivative as well as preparation method and application
A technology for medicines and compounds, applied in the field of honokiol derivatives and their preparation, can solve the problems of affecting drug efficacy, being insoluble in water, not easily absorbed and utilized, etc., and achieving the effects of good drug efficacy and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1 prepares honokiol sulfate and salt thereof
[0054] In 50ml of pyridine, add honokiol (cas35354-74-6, molecular weight 266) 10g, pyridine sulfur trioxide complex (cas26412-87-3, molecular weight 159) 17 grams (3 equivalents), stir overnight at room temperature, reduce Concentrate under pressure, add 50ml of dilute acid water, extract three times with 100ml of ethyl acetate, dry the ethyl acetate layer and concentrate to dryness to obtain 10.5 grams of honokiol disulfate as a white solid;
[0055] Add honokiol (cas35354-74-6, molecular weight 266) 10g, triethylamine sulfur trioxide complex (cas761-01-3, molecular weight 181.25) 19.4 grams (3 equivalents) in 50ml pyridine, stir overnight at room temperature , concentrated under reduced pressure, added 50ml dilute acid water, extracted three times with 100ml ethyl acetate, concentrated to dryness after the ethyl acetate layer was dried, and obtained 10.2 grams of honokiol disulfate as a white solid;
[0056] ...
Embodiment 2
[0073] Embodiment 2 prepares honokiol phosphate and salt thereof
[0074] Add honokiol (cas35354-74-6, molecular weight 266) 10g, phosphorus oxychloride (cas10025-87-3, molecular weight 153.33) 23 grams (4 equivalents) in 50ml pyridine, stir overnight at room temperature, concentrate under reduced pressure, Add 50ml of ice water and stir, concentrate under reduced pressure, add 50ml of acetone, a white solid precipitates, and filter to obtain 10.1 grams of honokiol bisphosphate;
[0075] Honokiol bisphosphate: 1 HNMR (300MHz,D 2 O): δ (ppm) 7.21-7.43 (m, 6H) 6.01 (brs, 2H), 4.96-5.07 (m, 4H), 3.33-3.66 (m, 4H);
[0076] ESI-MS m / z:425.2[M-H] - .
[0077] The structural formula of honokiol bisphosphate is as follows:
[0078]
[0079] Add honokiol (cas35354-74-6, molecular weight 266) 10g, phosphorus oxychloride (cas10025-87-3, molecular weight 153.33) 5.8 grams (1 equivalent) in 50ml pyridine, stir overnight at room temperature, concentrate under reduced pressure, Add...
Embodiment 3
[0092] The water solubility test of embodiment 3 honokiol sulfate, phosphoric acid ester and salt thereof
[0093] Detection method: Weigh the test sample ground into fine powder, put it in a certain volume of distilled water at 25°C±2°C, shake vigorously for 30s every 5min, observe the dissolution within 30min, if there are no visible solute particles or droplets, it is considered to be completely dissolved.
[0094] Very soluble means that 1g (ml) of solute can be dissolved in less than 1ml of solvent;
[0095] Soluble means that 1g (ml) of solute can be dissolved in 1 to less than 10ml of solvent;
[0096] Dissolution means that 1 g (ml) of solute can be dissolved in 10 to less than 30 ml of solvent;
[0097] Slightly soluble means that 1g (ml) of solute can be dissolved in 30 to less than 100ml of solvent;
[0098] Slightly soluble means that lg (ml) of solute can be dissolved in 100 to less than 1000ml of solvent;
[0099] Very slightly soluble means that 1 g (ml) of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com