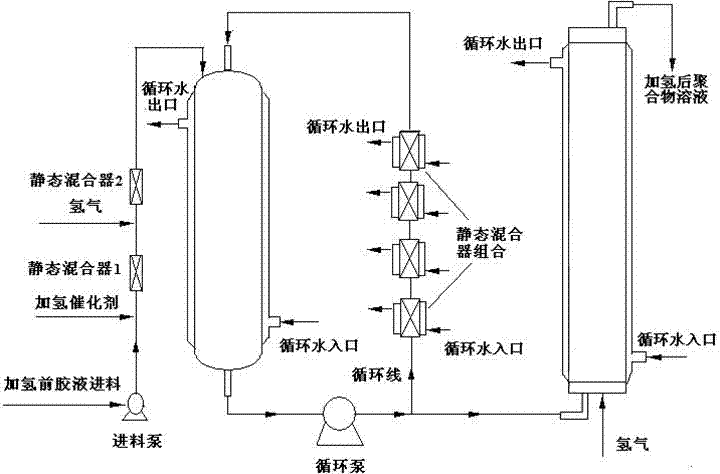
A hydrogenation method for a polymer comprising an olefin unsaturated bond
A hydrogenation reaction and unsaturated technology, which is applied in the field of polymer hydrogenation reaction containing olefinic unsaturated bonds, can solve the problems of hydrogenation efficiency, high double bond concentration, poor heat transfer effect of trickle bed reactor, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] This example is used to illustrate the hydrogenation method of polymers containing olefinic unsaturated bonds.
[0035] The 500L jacketed stirred reactor was fully replaced with refined nitrogen, and after the replacement, 250L of cyclohexane and n-hexane mixed solvent (the weight percentage of cyclohexane in the mixed solvent was 87% by weight) and 3.5L of refined styrene were added, and 1.15 L tetrahydrofuran, then add 0.71L of 0.5M n-butyllithium initiator solution (the molar ratio of tetrahydrofuran to n-butyllithium is 40:1), react at 50-60°C for 30 minutes, continue to add 24L refined butadiene to react for 40 Minutes, then add 3.5L refined styrene to react for 30 minutes, add 27ml isopropanol after the completion of the reaction to terminate the reaction. Here, the concentration of styrene-butadiene block copolymer (S-B-S) is about 10% by weight based on the entire polymerization system, the molecular weight of the block copolymer is 60,000, and the weight of sty...
Embodiment 2
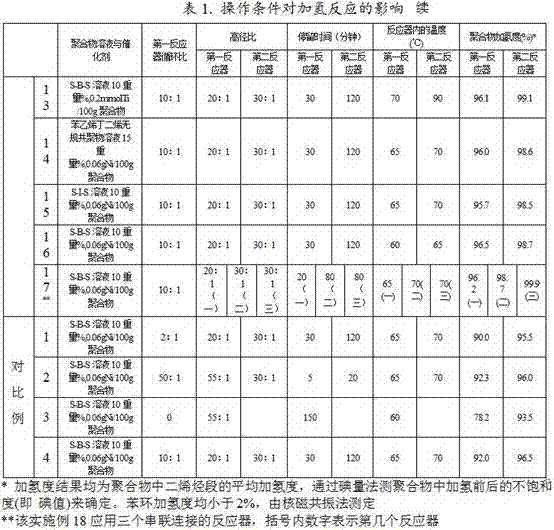
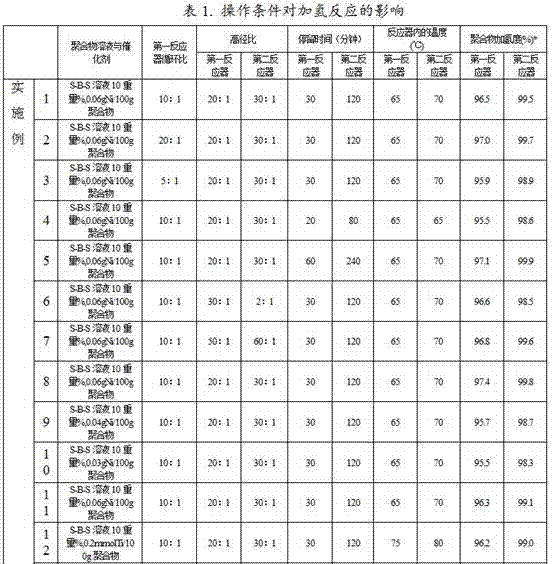
[0047]Except that the ratio of the circulating flow in the first reactor to the flow flowing out of the loop reactor is 20:1, the same method as in Example 1 is used to carry out the hydrogenation reaction containing the conjugated diene polymer solution, and the results are shown in Table 1.
Embodiment 3
[0049] Except that the ratio of the circulating flow in the first reactor to the flow flowing out of the loop reactor is 5:1, the same method as in Example 1 is used to carry out the hydrogenation reaction containing the conjugated diene polymer solution, and the results are shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com