Substituted bridged urea analogs as sirtuin modulators
A compound, heterocyclic technology, applied in the field of substituted bridged cyclic urea analogs as sirtuin modulators, can solve the problem of reducing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
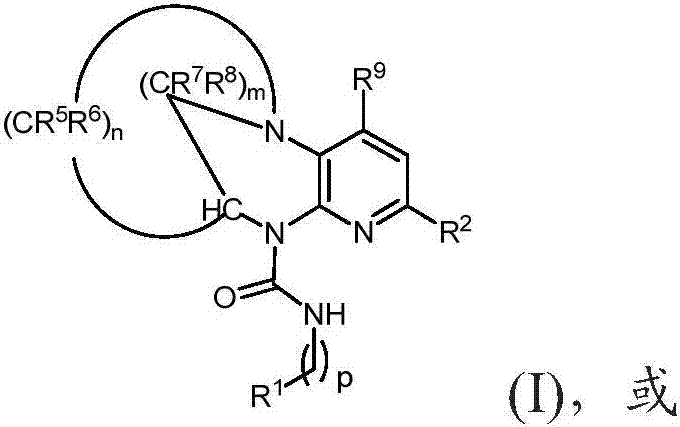
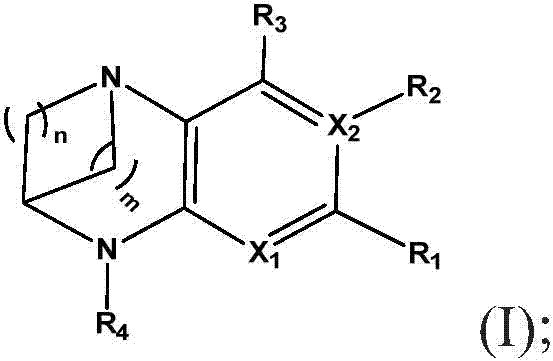
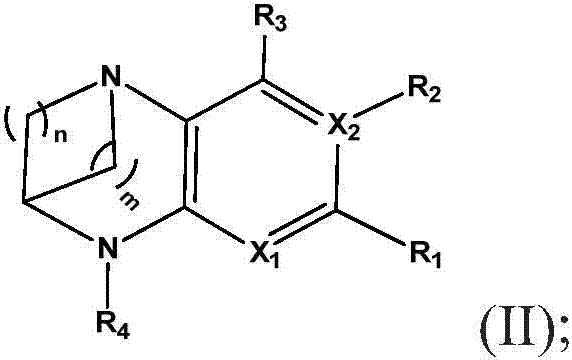
[1075] Synthesis of (9S)-N-(pyridin-2-yl)-2-((S)-2-(trifluoromethyl)pyrrolidin-1-yl)-8,9-dihydro-6H-5,9 -Methylenepyrido[2,3-b][1,4]diazacyclotetraene-10(7H)-carboxamide
[1076]
[1077] NaH (0.254 g, 10.57 mmol) was added to (9S)-2-((S)-2-(trifluoromethyl)pyrrolidin-1-yl)-7,8,9,10-tetrahydro-6H -5,9-methylenepyrido[2,3-b][1,4]diazaoctacene (0.550 g, 1.761 mmol) in tetrahydrofuran (THF) (30 mL) under nitrogen in a stirred solution at room temperature. The reaction mixture was stirred at room temperature for 30 minutes. Then 3-(pyridin-2-yl)-2H-pyrido[1,2-a][1,3,5]triazine-2,4(3H)-dione (0.635 g, 2.64 mmol ). The reaction mixture was then stirred at 65°C for 24 hours. The reaction mixture was cooled to 28 °C and partitioned between water (10 mL) and EtOAc (25 mL). The organic layer was separated and washed with anhydrous Na 2 SO 4 Dry, filter and evaporate the filtrate to give the crude material as a brown solid (TLC eluent: 100% EtOAc:R f -0.3; UV activity). The ...
Embodiment 2
[1080] Synthesis of (9S)-N-(5-fluoropyridin-3-yl)-2-(2-(trifluoromethyl)pyridin-4-yl)-8,9-dihydro-6H-5,9-ylidene Pyrido[2,3-b][1,4]diazacyclotetraene-10(7H)-carboxamide
[1081]
[1082] To (9S)-2-(2-(trifluoromethyl)pyridin-4-yl)-7,8,9,10-tetrahydro-6H-5,9-methylenepyrido[2 ,3-b][1,4]To a stirred solution of diazaoctene (500 mg, 1.561 mmol) in THF (15 mL) was added triethylamine (0.653 mL, 4.68 mmol) and triphosgene (232 mg, 0.780 mmol) and stirred at room temperature for 1 h. 5-Fluoropyridin-3-amine (525 mg, 4.68 mmol) was then added at 30°C and the reaction was heated at 70°C for 16h. THF was evaporated under reduced pressure and the resulting residue was diluted with water and washed with CH 2 Cl 2 (3x50 mL) extraction. The combined organic layers were washed with water, brine and washed with Na 2 SO 4 Drying and solvent evaporation under reduced pressure gave crude compound which was purified by flash column chromatography (silica gel: 100-200 mesh, eluent: 3% M...
Embodiment 3
[1085] Synthesis of (9S)-N-(pyrimidin-5-yl)-2-(5-(trifluoromethyl)pyridin-3-yl)-8,9-dihydro-6H-5,9-methylenepyridine And[2,3-b][1,4]diazacyclooctene-10(7H)-carboxamide
[1086]
[1087] To (9S)-2-(5-(trifluoromethyl)pyridin-3-yl)-7,8,9,10-tetrahydro-6H-5,9-methylenepyrido[2 , 3-b][1,4]diazaoctene (0.6g, 1.873mmol), triethylamine (0.783mL, 5.62mmol) in THF (12mL) was added triphosgene (0.278g , 0.937 mmol) and stirred at room temperature for 30 minutes. Pyrimidin-5-amine was then added and the reaction mixture was stirred at 65 °C for 16 h. The solvent was removed under reduced pressure to give the crude material which was diluted with dichloromethane, washed with water and brine solution and washed with anhydrous Na 2 SO 4 After drying, the organic layer was evaporated under reduced pressure to obtain crude product. The crude product mixture was purified by flash column chromatography (silica gel: 100-200 mesh, with CH 2 Cl 2 2% MeOH in ) to give (9S)-N-(pyrimidin-5-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com