A kind of levoxiracetam injection with few impurities and preparation method thereof
A technology with less impurities for injections, applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve problems such as large pH changes in solutions, poor stability, and increased impurities in products, and achieve Simple and feasible preparation process, good solubility and good clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A levoxiracetam injection with few impurities is prepared according to the following steps:
[0022] Element Dosage Levoxiracetam 100g glycerin 38g Glycine 29g Vitamin C 8g Ethylenediaminetetraacetic acid 6g Sterile water for injection Add to 1000ml
[0023] Made of 500
[0024] Preparation process:
[0025] 1. Concentrated preparation: Add the raw and auxiliary materials into the batching tank, then add 2 / 3 of the prescription amount of sterile water for injection, stir, dissolve, and obtain a concentrated preparation;
[0026] 2. Dilute preparation: take the concentrated preparation, add sodium phosphate salt buffer solution (accurately weigh 65.697g of disodium hydrogen phosphate and 2.346g of sodium dihydrogen phosphate, put it in a 1000ml volumetric flask, add purified water to dissolve, dilute to the volume , to obtain) adjust the pH to 6.8, add chitosan with a total volume of 0.2% to 0.6% mass volume ratio to ...
Embodiment 2
[0078] A levoxiracetam injection with few impurities is prepared according to the following steps:
[0079] Element Dosage Levoxiracetam 100g glycerin 30g Glycine 20g Vitamin C 7g Ethylenediaminetetraacetic acid 5g Sterile water for injection Add to 1000ml
[0080] Made of 500
[0081] Preparation process: prepared according to the preparation process of Example 1.
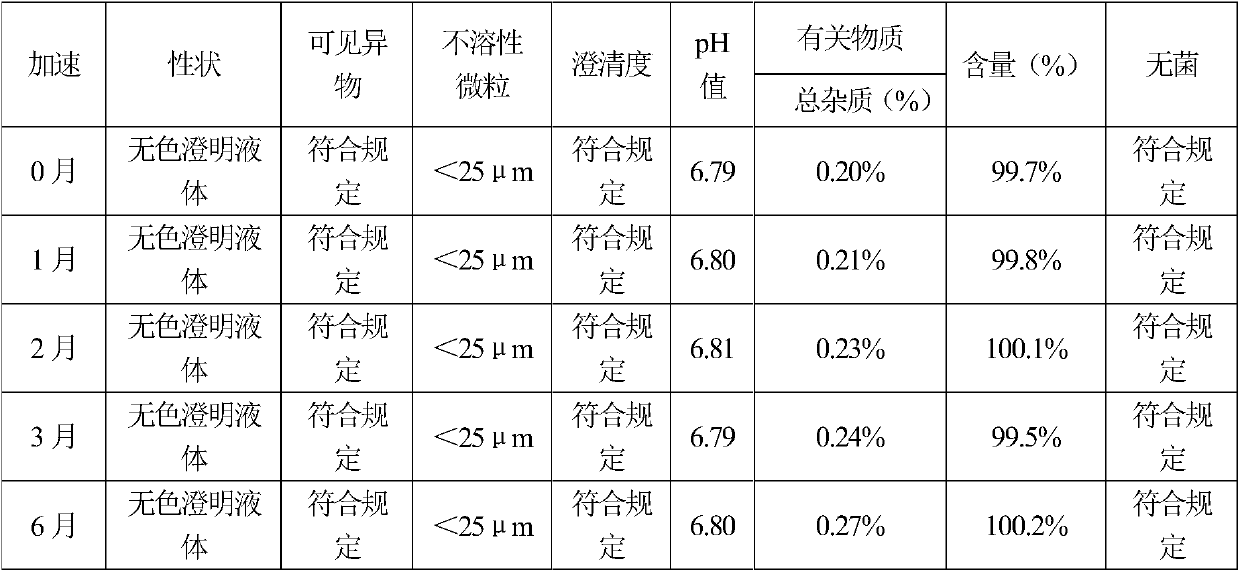
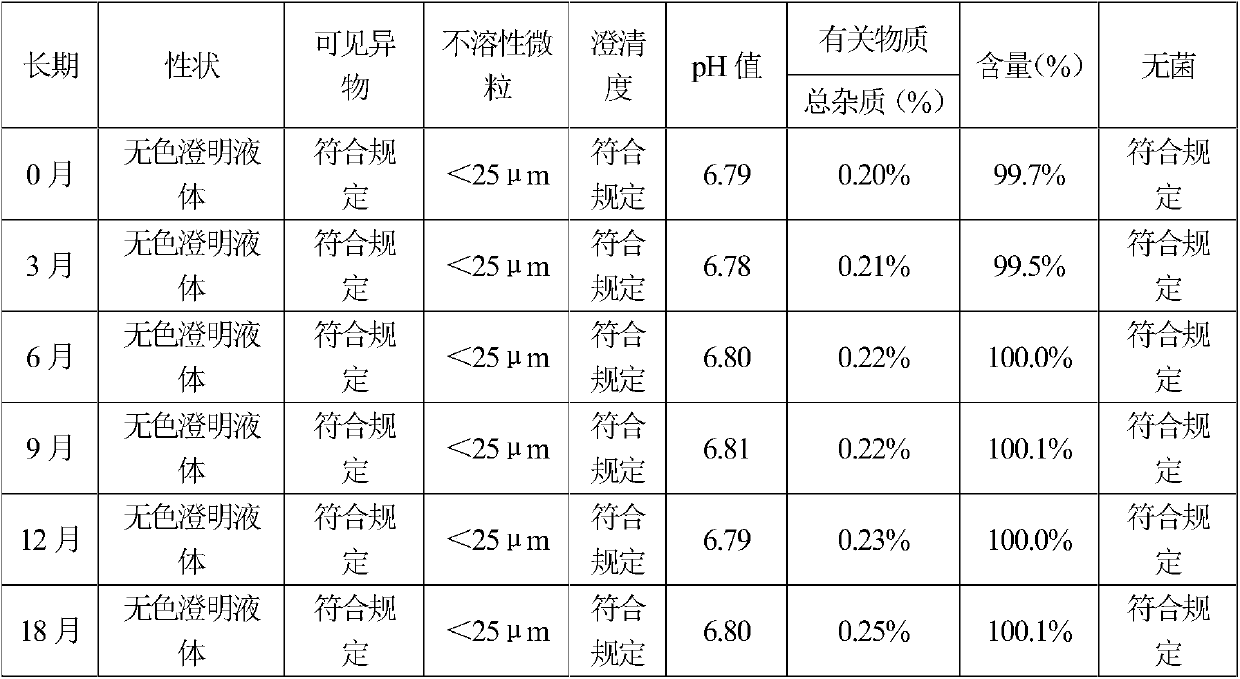
[0082] By the test method of embodiment 1, the sample stability test result of embodiment 2 shows that the sample quality is stable in accelerated June, and the quality is stable in long-term 18 months, so this product is valid for at least 18 months. The test results of the effect of sterilization process on the increase of impurities show that the prescription of Example 2, combined with a specific sterilization process, increases the related substances significantly better than that of the control sample. Clarity comparative test results show that the sample...
Embodiment 3
[0084] A levoxiracetam injection with few impurities is prepared according to the following steps:
[0085]
[0086]
[0087] Made of 500
[0088] Preparation process: prepared according to the preparation process of Example 1.
[0089] By the test method of embodiment 1, the sample stability test result of embodiment 3 shows that the sample quality is stable in accelerated June, and the long-term quality is stable in 18 months, so this product is valid for at least 18 months. The test results of the effect of sterilization process on the increase of impurities show that the prescription of Example 3, combined with a specific sterilization process, has significantly better increase of related substances than the control sample. Clarity comparative test results show that the sample clarity produced by Example 3 is less than No. 0.5 standard turbidity liquid, and this product has good clarity. Experiments on the influence of different pH regulators on the pH of the solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com